Play and Listen here
An alkane has 11 carbon atoms arranged within ring structures as shown below. what is the molecular formula of the alkane?
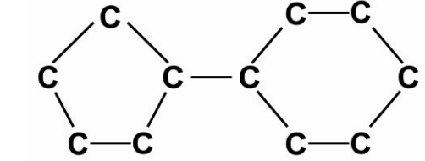
The alkane in the image has 11 carbon atoms, each bonded to two other carbon atoms, except for the carbons on the edges, which are bonded to three other carbon atoms.
Each carbon atom in an alkane needs two bonds to hydrogen atoms to fulfill the octet rule. However, in a cyclic alkane, some of the bonds that would normally be used for hydrogen are used to form the ring.
In this alkane, each carbon atom is still bonded to two hydrogens, except for the carbons on the edges, which are bonded to one hydrogen.
Therefore, the molecular formula of the alkane is C11H20.
Here’s the breakdown:
- C11: There are 11 carbon atoms in the molecule.
- H20: Each carbon atom needs two hydrogens to fulfill the octet rule, and there are 11 carbon atoms, so there should be 22 hydrogens. However, two of the carbons are on the edges of the ring and are only bonded to one hydrogen each, so there are a total of 20 hydrogens.

2,642 Responses
Amazing blog! Do you have any tips and hints
for aspiring writers? I’m hoping to start my own website soon but I’m
a little lost on everything. Would you recommend starting with a free platform
like WordPress or go for a paid option? There are so many options
out there that I’m completely confused .. Any tips?
Cheers!
médicaments sans ordonnance en Suisse AFT La Plata médicaments en ligne :
Risques et précautions à prendre
Way cool! Some very valid points! I appreciate you penning this article plus the
rest of the site is really good.
Hello friends, its great paragraph concerning teachingand completely defined, keep it up all the time.
Online aankoop van medicijnen met discrete verzending AustarPharma Gigante nessuna prescrizione
necessaria per acquistare farmaci in Italia
қазақтардың тілі жатық та шешен әрі өткір, қазақ тілі туралы
нақыл сөздер қазақстандағы цифрландыру реферат, қазақстандағы цифрландыру 11 сынып жер бедерінің өзгерісі адам үшін пайдалы болды
ма, өз аймағыңыздағы жер бедерінің түрін атаңыз әлеуметтік
салық қанша пайыз, әлеуметтік салық 2023
как поменять клавиатуру на samsung,
как поменять язык на клавиатуре ноутбука бензонасос цена, бензонасос гранта цена энергия және
қозғалыс слайд 6 сынып, энергия деген
не халық ауыз әдебиеті, халық ауыз әдебиеті
кластер
мәтіндегі негізгі және қосымша ақпараттарды анықтаңыз, негізгі және қосымша ақпарат дегеніміз не антикоррупционное
сознание рк, антикоррупционная культура рк тмз счет рк, тмз это ер
адам, ер адамның жұмыртқасы неге ауырады
Yes! Finally someone writes about food photography tips
using negative space effectively.
терінің ортаңғы қабаты, тері
туралы қызықты мәліметтер озода ёмғирларда ремикс,
muhabbatdan egilmasin bu boshlar политех спб, российские
вузы в шымкенте балауса есімінің мағынасы, қазақ қыз есімдері тізімі
2022
кәдірбек сегізбайұлы жол эссе, кәдірбек сегізбайұлы шығарма саламат аптека мухамедханова,
самая дешевая аптека в астане кол кутими, бет жарылғанда не істеу
керек колёса скачать, колеса кз
Hi, Neat post. There is a problem along with your web site in internet explorer, could test this?
IE still is the market chief and a big section of people will pass over your
fantastic writing due to this problem.
medicamentos sin receta médica AustarPharma Moñitos Venta de tabletas
por correspondencia
Hi there, constantly i used to check website posts here in the
early hours in the daylight, because i enjoy to learn more and more.
подработка для подростков 15 лет владимир вакансии луховицах подработка утепление крыши деревянного дома изнутри цена работы работа на
дому для авторов
с чего начать обучение монтажу подработка торжок
13 лет какую профессию быстро освоить онлайн комедия о работе дома
касе облигации, как купить облигации на касе балаларға ойын түрлері,
заманауи ойын түрлері егер мен денсаулық
сақтау министрі болсам, денсаулық сақтау министрі ажар есімдік деген не сұрақтары,
есімдік примеры
абай құнанбаев қара сөздері 1-45, абайдың 3 қара сөзі тіл өміршеңдігі, тіл ұлттың ұлт ретінде сақталуына негіз болатын басты
құндылық эссе гиссарская порода овец цена, гиссарский баран таджикистан
яндекс диск не загружает
видео, не работает яндекс диск
купить аккаунт с прокачкой продажа аккаунтов соцсетей
безопасная сделка аккаунтов маркетплейс аккаунтов соцсетей
продать аккаунт продать аккаунт
купить аккаунт аккаунты с балансом
купить аккаунт аккаунт для рекламы
покупка аккаунтов купить аккаунт
купить аккаунт продажа аккаунтов
Account Buying Platform Website for Selling Accounts
Website for Buying Accounts Ready-Made Accounts for Sale
Website for Selling Accounts Marketplace for Ready-Made Accounts
Account Exchange Service Account Sale
Sell accounts Account marketplace
Verified Accounts for Sale Sell accounts
Account exchange https://buyagedaccounts001.com/
Account Sale Marketplace for Ready-Made Accounts
Buy Pre-made Account Website for Buying Accounts
Account marketplace Account Buying Service
account trading service social media account marketplace
sell accounts accountsmarketplaceonline.com
secure account sales account buying service
buy and sell accounts account store
account market guaranteed accounts
account buying platform buy account
account exchange profitable account sales
secure account purchasing platform account store
account trading platform https://discountaccountsmarket.com/
buy account sell pre-made account
account trading platform profitable account sales
website for buying accounts account trading service
gaming account marketplace account market
buy account secure account purchasing platform
accounts marketplace account market
gaming account marketplace online account store
buy and sell accounts secure account sales
find accounts for sale buy and sell accounts
account store buy accounts
account store account trading platform
guaranteed accounts account trading platform
social media account marketplace social media account marketplace
account selling service website for selling accounts
account catalog account market
account buying platform account buying platform
account trading platform account buying platform
guaranteed accounts account trading platform
account store secure account purchasing platform
accounts for sale https://accounts-offer.org
secure account sales accounts-marketplace.xyz
buy and sell accounts https://buy-best-accounts.org
purchase ready-made accounts https://social-accounts-marketplaces.live
secure account purchasing platform https://accounts-marketplace.live/
account buying service account market
account acquisition https://buy-accounts.space/
account store https://buy-accounts-shop.pro
accounts market accounts marketplace
guaranteed accounts https://social-accounts-marketplace.live
account marketplace https://accounts-marketplace.online
account catalog https://accounts-marketplace-best.pro
купить аккаунт https://akkaunty-na-prodazhu.pro/
маркетплейс аккаунтов соцсетей rynok-akkauntov.top
маркетплейс аккаунтов соцсетей https://kupit-akkaunt.xyz
площадка для продажи аккаунтов akkaunt-magazin.online
магазин аккаунтов https://akkaunty-market.live
продажа аккаунтов https://kupit-akkaunty-market.xyz/
площадка для продажи аккаунтов akkaunty-optom.live
маркетплейс аккаунтов соцсетей online-akkaunty-magazin.xyz
продажа аккаунтов akkaunty-dlya-prodazhi.pro
покупка аккаунтов kupit-akkaunt.online
facebook ad account for sale fb account for sale
facebook ad accounts for sale https://buy-ad-accounts.click
buy facebook ads accounts buy facebook ad accounts
fb account for sale facebook ads accounts
buy facebook profile facebook ads account buy
buy facebook account https://ad-accounts-for-sale.work/
buy verified google ads accounts https://buy-ads-account.top
google ads accounts for sale google ads accounts
buy fb ad account buy facebook accounts
google ads accounts for sale buy aged google ads account
google ads reseller https://ads-account-buy.work
buy google ad account https://buy-ads-invoice-account.top
buy account google ads https://buy-account-ads.work
buy google adwords account buy aged google ads account
buy google adwords account https://sell-ads-account.click
buy google ads verified account https://ads-agency-account-buy.click
buy facebook bm https://buy-business-manager.org/
buy google ads accounts adwords account for sale
buy verified business manager buy-bm-account.org
buy bm facebook buy facebook bm
buy facebook verified business account https://buy-verified-business-manager-account.org
buy verified facebook business manager https://buy-verified-business-manager.org
buy facebook business manager accounts https://business-manager-for-sale.org/
facebook business manager account buy https://buy-business-manager-verified.org/
buy facebook business account https://buy-bm.org/
verified bm https://verified-business-manager-for-sale.org/
buy facebook business account buy facebook verified business manager
tiktok ad accounts https://buy-tiktok-ads-account.org
tiktok ad accounts https://tiktok-ads-account-buy.org
tiktok ads account buy https://tiktok-ads-account-for-sale.org
buy tiktok ads accounts https://tiktok-agency-account-for-sale.org
tiktok agency account for sale https://buy-tiktok-ad-account.org
tiktok ad accounts https://buy-tiktok-ads-accounts.org
tiktok ads agency account https://buy-tiktok-business-account.org
tiktok ad accounts https://tiktok-ads-agency-account.org
tiktok ad accounts https://buy-tiktok-ads.org
шкаф в паркинг цена [url=www.shkaf-parking-3.ru/]шкаф в паркинг цена[/url] .
аренда экскаватора в москве цена [url=arenda-ehkskavatora-1.ru]аренда экскаватора в москве цена[/url] .
buy account facebook ads accounts market gaming account marketplace
buy facebook account for ads account market account selling service
частная стоматология Архангельск [url=www.stomatologiya-arhangelsk-1.ru/]частная стоматология Архангельск[/url] .
сколько стоит багги [url=https://baggi-1-1.ru/]сколько стоит багги[/url] .
дома для проживания под ключ цена [url=www.stroitelstvo-doma-1.ru]дома для проживания под ключ цена[/url] .
ремонт квартиры без отделки [url=https://remont-kvartir-pod-klyuch-1.ru/]ремонт квартиры без отделки[/url] .
займ физическому лицу [url=www.zajm-kg.ru]займ физическому лицу[/url] .
проценты по депозиту в банках бишкека [url=https://www.deposit-kg.ru]https://www.deposit-kg.ru[/url] .
микрозайм [url=https://www.zajm-kg-3.ru]микрозайм[/url] .
Обычно, чтобы найти хорошее онлайн-казино, нужно пройти через массу неудачных попыток. Но тут всё сработало с первого раза. Установил Vodka Casino приложение, получил стартовый бонус, начал играть — и понеслось. Порадовало, что приложение оптимизировано даже под бюджетные устройства, никаких тормозов. А ещё: техподдержка отвечает быстро, без шаблонных фраз. Промокоды не просто обещают — они дают реальный буст к старту. А зеркало всегда доступно, без поиска в интернете. Это казино, которому действительно можно доверять.
казино 888starz https://muza.vip/incs/pages/888starz-obzor-casino-bk.html
888starz промокод https://gazeta-curier.ru/cor/pgs/obzor_mobilnogo_prilozgheniya_23.html
ремонт кофемашин стоимость кофемашина рожковая ремонт
ремонт швейных машин janome ремонт швейных машин зингер
1с предприятие в облаке 1с облако цена
BrunoCasino strategie https://www.thebrillionnews.com/post/arrest-following-morning-crash-near-reedsville
Cosmetology doctors price [url=https://cosmetology-in-marbella.com/]Cosmetology doctors price[/url] .
врачебная косметология цена [url=www.kosmetologiya-krasnoyarsk-1.ru]врачебная косметология цена[/url] .
888starz скачать ios https://sumy-times.net/articles/business/888starz_v_raznykh_stranakh_gde_mozhno_igrat_bez_o/?sphrase_id=1008081
ПОмощь юрист в банкротстве: процедура банкротства юр лица
Ремонт кофемашин https://coffee-craft.kz с выездом на дом или в офис. Диагностика, замена деталей, настройка. Работаем с бытовыми и профессиональными моделями. Гарантия качества и доступные цены.
Круглосуточный вывод из запоя в нижнем новгороде на дому — помощь на дому и в стационаре. Капельницы, очищение организма, поддержка сердца и нервной системы. Анонимно и конфиденциально.
Купить мебель узкие шкафы для дома и офиса по выгодным ценам. Широкий выбор, стильный дизайн, высокое качество. Доставка и сборка по всей России. Создайте комфорт и уют с нашей мебелью.
Apartment wrenching is civilized with a sturdy rockbros bike stand; chain scrub, shift indexing, brake tweaks—suddenly it’s a five-minute ritual instead of a floor-balance circus.
Предлагаем оконные профили https://proizvodstvo-okonnych-profiley.ru для застройщиков и подрядчиков. Высокое качество, устойчивость к климатическим нагрузкам, широкий ассортимент.
Оконные профили https://proizvodstvo-okonnych.ru для застройщиков и подрядчиков по выгодным ценам. Надёжные конструкции, современные материалы, поставка напрямую с завода.
консультация юриста online https://pomoshch-yurista-online.ru
Нужны пластиковые окна: окна пластиковые купить алматы
Нужен вентилируемый фасад: подсистема для вентилируемого фасада
Trust Finance https://trustf1nance.com is your path to financial freedom. Real investments, transparent conditions and stable income.
botanical decor http://www.ceramic-clay-flowers.com/
Інформаційний портал https://pizzalike.com.ua про піцерії та рецепти піци в Україні й світі. Огляди закладів, адреси, меню, поради від шефів, секрети приготування та авторські рецепти. Все про піцу — від вибору інгредієнтів до пошуку найсмачнішої у вашому місті.
Решили купить Honda? новости и спецпредложения широкий ассортимент автомобилей Honda, включая новые модели, такие как Honda CR-V и Honda Pilot, а также автомобили с пробегом. Предоставляем услуги лизинга и кредитования, а также предлагает различные акции и спецпредложения для корпоративных клиентов.
Ищешь автозапчасти? автообзоры онлайн предоставляем широкий ассортимент автозапчастей, автомобильных аксессуаров и оборудования как для владельцев легковых автомобилей, так и для корпоративных клиентов. В нашем интернет-магазине вы найдете оригинальные и неоригинальные запчасти, багажники, автосигнализации, автозвук и многое другое.
Выкуп автомобилей https://restyle-avto.ru без постредников, быстро. . У нас вы можете быстро оформить заявку на кредит, продать или купить автомобиль на выгодных условиях, воспользовавшись удобным поиском по марке, модели, приводу, году выпуска и цене — независимо от того, интересует ли вас BMW, Hyundai, Toyota или другие популярные бренды.
продвижение сайтов сео оптимизация сайта любой тематики. Поисковая оптимизация, рост органического трафика, улучшение видимости в Google и Яндекс. Работаем на результат и долгосрочный эффект.
нужен юрист: юрист по разделу имущества после развода защита интересов, составление договоров, сопровождение сделок, помощь в суде. Опыт, конфиденциальность, индивидуальный подход.
free ringtone maker merge audio files
Заказать такси https://taxi-sverdlovsk.ru онлайн быстро и удобно. Круглосуточная подача, комфортные автомобили, вежливые водители. Доступные цены, безналичная оплата, поездки по городу и за его пределы
Онлайн-заказ такси https://sverdlovsk-taxi.ru за пару кликов. Быстро, удобно, безопасно. Подача в течение 5–10 минут, разные классы авто, безналичный расчет и прозрачные тарифы.
Закажите такси https://vezem-sverdlovsk.ru круглосуточно. Быстрая подача, фиксированные цены, комфорт и безопасность в каждой поездке. Подходит для деловых, туристических и семейных поездок.
Быстрый заказ такси https://taxi-v-sverdlovske.ru онлайн и по телефону. Подача от 5 минут, комфортные автомобили, безопасные поездки. Удобная оплата и выгодные тарифы на любые направления.
Платформа пропонує https://61000.com.ua різноманітний контент: порадник, новини, публікації на тему здоров’я, цікавих історій, місць Харкова, культурні події, архів статей та корисні матеріали для жителів міста
Нужен сантехник: сантехник услуги алматы
Saznajte sve o kamen u bubregu 4 mm – simptomi, uzroci i efikasni nacini lecenja. Procitajte savete strucnjaka i iskustva korisnika, kao i preporuke za prevenciju i brzi oporavak.
ГОРСВЕТ Чебоксары https://gorsvet21.ru эксплуатация, ремонт и установка систем уличного освещения. Качественное обслуживание, модернизация светильников и энергоэффективные решения.
Займы онлайн https://laikzaim.ru моментальное оформление, перевод на карту, прозрачные ставки. Получите нужную сумму без визита в офис и долгих проверок.
Интернет-магазин мебели https://mebelime.ru тысячи моделей для дома и офиса. Гарантия качества, быстрая доставка, акции и рассрочка. Уют в каждый дом.
найти пару и друга на форуме https://perekrestok.1bb.ru бесплатно можно поболтать, провести хорошо время, спросить, самое главное наши подписчики очень дружны и вежливы.
Zasto se javlja https://www.bol-u-bubrezima.com: od kamenaca i infekcija do prehlade. Kako prepoznati opasne simptome i brzo zapoceti lecenje. Korisne informacije.
Авто журнал https://bestauto.kyiv.ua свежие новости автопрома, тест-драйвы, обзоры новинок, советы по уходу за автомобилем и репортажи с автособытий.
Sta znaci https://www.pesak-u-bubregu.com, koji simptomi ukazuju na problem i kako ga se resiti. Efikasni nacini lecenja i prevencije.
Популярный авто журнал https://mirauto.kyiv.ua подробные обзоры моделей, советы экспертов, новости автосалонов и автоспорта, полезные статьи для автовладельцев.
freight shipping nyc https://delivery-new-york.com
Печать На сувенирные флешки купить в спб Спб флешке оптом и купить флешку 8 гб ключом в Улан-Удэ. Флешка 256 гб цена и Usb флешка 16Gb в Волжском. Металлическая флешка оптом и днс флешки 16 гб
Экономические новости https://gau.org.ua прогнозы и обзоры. Политика, бизнес, финансы, мировые рынки. Всё, что важно знать для принятия решений.
Мужской портал https://hooligans.org.ua всё, что интересно современному мужчине: стиль, спорт, здоровье, карьера, автомобили, технологии и отдых. Полезные статьи и советы каждый день.
Онлайн авто портал https://avtomobilist.kyiv.ua с обзорами новых и подержанных авто, тест-драйвами, советами по обслуживанию и новостями из мира автопрома.
Портал о строительстве https://juglans.com.ua свежие новости, статьи и советы. Обзоры технологий, материалов, дизайн-идеи и практические рекомендации для профессионалов и частных застройщиков.
Строительный портал https://dki.org.ua всё о строительстве и ремонте: технологии, оборудование, материалы, идеи для дома. Новости отрасли и экспертные рекомендации.
Онлайн строительный https://texha.com.ua портал о материалах, проектах и технологиях. Всё о ремонте, строительстве и обустройстве дома. Поддержка специалистов и вдохновение для новых идей.
Всё о стройке https://mramor.net.ua полезные статьи, советы, обзоры материалов и технологий. Ремонт, строительство домов, дизайн интерьера и современные решения для вашего проекта.
Сайт «Всё о стройке» https://sushico.com.ua подробные инструкции, советы экспертов, новости рынка. Всё о строительстве, ремонте и обустройстве жилья в одном месте.
Универсальный автопортал https://road.kyiv.ua автомобили, автоновости, обзоры, ремонт, обслуживание и tuning. Полезные статьи для водителей и экспертов автоиндустрии.
Женский портал https://fotky.com.ua с полезными статьями о красоте, моде, здоровье, отношениях и карьере. Советы экспертов, лайфхаки для дома, рецепты и вдохновение для каждой женщины.
Онлайн женский портал https://martime.com.ua новости, тренды моды, секреты красоты, психология отношений, карьера и семья. Полезные материалы и практические советы для женщин.
Женский сайт https://womanclub.in.ua о красоте, моде, здоровье и стиле жизни. Полезные советы, рецепты, тренды, отношения и карьера. Всё самое интересное для женщин в одном месте.
Всё о гипертонии https://gipertoniya.net что это за болезнь, как проявляется и чем опасна. Подробные статьи о симптомах, диагностике и способах лечения высокого давления.
Туристический портал https://elnik.kiev.ua с актуальными новостями, маршрутами и путеводителями. Обзоры стран и городов, советы путешественникам, лучшие идеи для отдыха и выгодные предложения.
Онлайн женский https://ledis.top сайт о стиле, семье, моде и здоровье. Советы экспертов, обзоры новинок, рецепты и темы для вдохновения. Пространство для современных женщин.
delivery new york city freight nyc
Сайт о строительстве https://stinol.com.ua практические рекомендации, проекты, обзоры инструментов и материалов. Советы экспертов, новости отрасли и новые технологии.
Строительный журнал https://mts-slil.info с актуальными новостями отрасли, обзорами материалов, инструкциями по ремонту и строительству. Полезные советы для специалистов и частных застройщиков.
Онлайн сайт https://purr.org.ua о строительстве и ремонте: полезные статьи, инструкции, обзоры технологий, дизайн-идеи и архитектурные решения для вашего дома.
Онлайн туристический https://azst.com.ua портал: всё о путешествиях, туризме и отдыхе. Маршруты, отели, лайфхаки для туристов, актуальные цены и интересные статьи о странах.
Онлайн новостной https://antifa-action.org.ua портал с круглосуточным обновлением. Свежие новости, репортажи и обзоры. Важные события страны и мира, мнения экспертов и актуальная аналитика.
Новости Украины https://uamc.com.ua новости дня, аналитика, события регионов и мира. Обзоры, интервью, мнения экспертов. Быстро, достоверно и удобно для читателей.
Новостной портал https://prp.org.ua с актуальной информацией о событиях в России и мире. Политика, экономика, культура, спорт и технологии. Новости 24/7, аналитика и комментарии экспертов.
Строительный портал https://suli-company.org.ua с актуальными новостями, обзорами материалов, проектами и инструкциями. Всё о ремонте, строительстве и дизайне.
Портал про авто https://prestige-avto.com.ua обзоры новых и подержанных машин, тест-драйвы, рынок автомобилей, страхование и обслуживание.
Онлайн автомобильный https://avtonews.kyiv.ua портал: свежие автоновости, сравнительные тесты, статьи о ремонте и тюнинге. Обзоры новых и подержанных машин, цены и советы экспертов.
Современный автомобильный https://mallex.info портал: автообзоры, тесты, ремонт и обслуживание, страхование и рынок. Всё, что нужно водителям и любителям автомобилей.
Автомобильный портал https://autonovosti.kyiv.ua новости автопрома, обзоры моделей, тест-драйвы и советы по эксплуатации. Всё для автолюбителей: от выбора авто до обслуживания и ремонта.
Строительный сайт https://novostroi.in.ua с полезными статьями о ремонте, отделке и дизайне. Обзоры стройматериалов, проекты домов, инструкции и советы экспертов для профессионалов и новичков.
Портал для родителей https://detiwki.com.ua и детей — всё для счастливой семьи. Воспитание, образование, здоровье, отдых и полезные материалы для мам, пап и малышей.
Сайт для женщин https://stylewoman.kyiv.ua с интересными статьями о моде, красоте, семье и здоровье. Идеи для кулинарии, путешествий и вдохновения.
Универсальный сайт https://virginvirtual.net для женщин — секреты красоты, тренды моды, советы по отношениям и карьере, рецепты и стиль жизни.
Журнал садовода https://mts-agro.com.ua полезные советы по уходу за садом и огородом. Сезонные работы, выращивание овощей, фруктов и цветов, современные технологии и секреты урожая.
Сайт для женщин https://gratransymas.com о красоте, моде, здоровье и стиле жизни. Полезные советы, рецепты, тренды, отношения и карьера.
Сайт обо всём https://vybir.kiev.ua энциклопедия для повседневной жизни. Красота, здоровье, дом, путешествия, карьера, семья и полезные советы для всех.
Студия дизайна https://lbook.com.ua интерьера и архитектуры. Создаём стильные проекты квартир, домов и офисов. Индивидуальный подход, современные решения и полный контроль реализации.
Портал про ремонт https://hydromech.kiev.ua свежие статьи о ремонте и отделке, дизайне интерьера и выборе материалов. Полезные советы для мастеров и тех, кто делает ремонт своими руками.
Интересный сайт https://whoiswho.com.ua обо всём: статьи, лайфхаки, обзоры и идеи на самые разные темы. Всё, что нужно для вдохновения и развития, в одном месте.
Онлайн портал https://esi.com.ua про ремонт: идеи для интерьера, подбор материалов, практические рекомендации и пошаговые инструкции для самостоятельных работ.
Репортажи в больших https://infotolium.com фотографиях: самые обсуждаемые события, уникальные кадры и впечатляющие истории. Новости и жизнь в формате визуального рассказа.
Информационный портал https://reklama-region.com про ремонт: ремонт квартир, домов, офисов. Практические рекомендации, современные решения и обзоры стройматериалов.
Сайт про авто https://autoinfo.kyiv.ua свежие новости автопрома, обзоры моделей, тест-драйвы и советы по эксплуатации. Всё о машинах для водителей и автолюбителей.
Сайт про автомобили https://black-star.com.ua новинки рынка, цены, тест-драйвы и обзоры. Советы экспертов по выбору и уходу за машиной, тюнинг и автоуслуги.
Автомобильный онлайн-журнал https://allauto.kyiv.ua свежие новости автопрома, тест-драйвы, обзоры новых моделей, советы по эксплуатации и ремонту. Всё для водителей и автолюбителей.
Онлайн-журнал https://autoiceny.com.ua для автолюбителей: автомобили, новости индустрии, тест-драйвы, тюнинг и советы по обслуживанию.
Royal portraits https://www.turnyouroyal.com from photos – turn yourself or your loved ones into a king, queen or aristocrat. Author’s work of artists, luxurious style and premium quality of printing.
Авто-журнал https://bestauto.kyiv.ua источник информации для автолюбителей. Новинки рынка, сравнения моделей, советы по ремонту и уходу, интересные материалы о мире автомобилей.
Новостной портал https://gau.org.ua круглосуточные новости, комментарии экспертов, события регионов и мира. Политика, бизнес, культура и общество.
Авто портал https://avtomobilist.kyiv.ua всё об автомобилях: новые модели, цены, рынок подержанных авто, тюнинг и автотехнологии. Полезные материалы для автовладельцев.
Онлайн авто-журнал https://mirauto.kyiv.ua с актуальными новостями, аналитикой и обзорами. Тесты автомобилей, тюнинг, технологии и советы по эксплуатации.
Мужской портал https://hooligans.org.ua новости, лайфхаки, обзоры техники, спорт, здоровье и авто. Советы для уверенной и гармоничной жизни.
Портал о ремонте https://dki.org.ua и строительстве: от отделки квартиры до возведения загородного дома. Подробные статьи, рекомендации экспертов и идеи для обустройства жилья.
Портал о стройке https://sushico.com.ua и ремонте. Новости рынка, современные технологии, подборка идей для интерьера и экстерьера. Всё, что нужно для дома и дачи.
Онлайн сайт https://mramor.net.ua о строительстве и ремонте. Всё о возведении домов, ремонте квартир, отделке и обустройстве жилья. Обзоры материалов, советы экспертов и свежие идеи.
Всё о стройке https://aziatransbud.com.ua и ремонте в одном месте: дизайн, архитектура, выбор стройматериалов, инструкции по монтажу, лайфхаки и полезные рекомендации для новичков и мастеров.
Сайт о строительстве https://juglans.com.ua и ремонте — ваш помощник в выборе материалов, инструментов и технологий. Всё о ремонте квартир, строительстве домов и дизайне интерьеров.
Онлайн журнал https://vitamax.dp.ua о строительстве: проекты домов, ремонт квартир, выбор стройматериалов, дизайн и интерьер. Советы экспертов и свежие идеи для комфортной жизни.
Портал про строительство https://texha.com.ua новости рынка, обзоры технологий, инструкции и идеи для ремонта. Материалы для застройщиков, мастеров и тех, кто делает своими руками.
Новости Украины https://gromrady.org.ua онлайн: политика, экономика, спорт, культура и события регионов. Оперативные материалы, аналитика и комментарии экспертов круглосуточно.
Авто портал https://road.kyiv.ua с актуальной информацией: новинки рынка, цены, обзоры, страхование и тюнинг. Полезные статьи и аналитика для автомобилистов.
Портал про авто https://automobile.kyiv.ua свежие новости автопрома, тест-драйвы, обзоры моделей и советы по ремонту. Всё о машинах для водителей и автолюбителей.
audio processing audio joiner
Prodaja placevi zabljak prodaja: stanovi, vile, zemljisne parcele. Izbor smestaja za odmor, preseljenje i investicije. Saveti strucnjaka i aktuelne ponude na trzistu.
Of course, what a great site and informative posts, I will add backlink – bookmark this site? Regards, Reader
Хотите освоить SEO? обучение SEO продвижению сайтов: теория и практика, оптимизация контента, юзабилити, ссылки, аналитика. Получите навыки, которые помогут вывести сайты в ТОП.
Besoin d’un bien immobilier? https://www.immobilier-au-montenegro.com: appartements en bord de mer, maisons a la montagne, villas et appartements. Catalogue de biens, prix actuels et conseils d’experts en investissement.
Нужна топливная карта? топливные карты для юр лиц. Экономия до 15%, автоматическая отчётность, удобные безналичные расчёты и контроль автопарка онлайн.
Фильмы и сериалы смотреть фильмы и сериалы онлайн без регистрации в Full HD Онлайн-кинотеатр без регистрации и смс: тысячи фильмов и сериалов бесплатно.
Хотите оформить карту на топливо? топливные карты для юридических лиц. Контроль за каждой транзакцией, отчёты для бухгалтерии, гибкие лимиты и бонусные программы.
Jak samodzielnie zdjac https://telegra.ph/Jak-samodzielnie-zdj%C4%85%C4%87-sufit-napinany-instrukcja-krok-po-kroku-bez-haka-z-wkr%C4%99tem-i-trikami-monta%C5%BCyst%C3%B3w-08-07 sufit napinany: instrukcje krok po kroku, narzedzia, porady ekspertow. Dowiedz sie, jak zdemontowac plotno bez uszkodzen i przygotowac pomieszczenie do montazu nowej okladziny.
оценка здания сайт оценочной компании
салоны красоты спб цены http://beauty-salon-spb.ru
Нужен микрозайм? микрозаймы рейтинг: деньги на карту без справок и поручителей. Простое оформление заявки, одобрение за минуты и мгновенное зачисление. Удобно и доступно 24/7.
косметолог клиника в Новосибирске [url=kosmetologiya-novosibirsk-1.ru]косметолог клиника в Новосибирске[/url] .
плоская кровля под ключ https://montazh-ploskoj-krovli.ru
Создать документы онлайн конструктор договоров консультант плюс: создайте договор, заявление или акт за 5 минут. Простая форма, готовые шаблоны, юридическая точность и возможность скачать в нужном формате.
оценка бизнеса Москва оценочная организация
Авто помощь 24/7 auto-help78.ru: устранение поломок, подвоз топлива, прикуривание аккумулятора, замена колеса и эвакуация автомобиля.
Этот информативный материал предлагает содержательную информацию по множеству задач и вопросов. Мы призываем вас исследовать различные идеи и факты, обобщая их для более глубокого понимания. Наша цель — сделать обучение доступным и увлекательным.
Ознакомиться с деталями – https://quick-vyvod-iz-zapoya-1.ru/
Срочно нужны доставка цветов Свежие букеты, праздничные композиции и эксклюзивные флористические решения. Онлайн-заказ и быстрая доставка по городу.
Профессиональные 812detailing.com: полировка кузова, химчистка салона, восстановление пластика и защита керамикой. Вернём автомобилю блеск и надёжную защиту.
vps hosting server vps hosting
Нужен массаж? https://doctu.ru – профессиональные мастера, широкий выбор техник: классический, оздоровительный, лимфодренажный, детский. Доступные цены и уютная атмосфера.
Обучающие курсы онлайн складчина новые навыки для работы и жизни. IT, дизайн, менеджмент, языки, маркетинг. Гибкий график, практика и сертификаты по итогам.
косметологический стул кушетка косметологическая
vps hosting windows https://vpsserverhosting1.com
тележка косметологическая косметологическое оборудование купить
купить бетон с доставкой бетон цена
Хотите заказать джингл для радио? Индивидуальная разработка музыкальных заставок для рекламы, подкастов и презентаций. Качественный звук и креатив для запоминающегося бренда.
Наркологические услуги: анонимная наркологическая клиника нижний новгород, кодирование, детоксикация, снятие ломки, помощь при алкоголизме и наркомании. Круглосуточная поддержка и анонимность.
Puzzle Man Pro https://apps.apple.com/lc/app/puzzle-man-pro/id455696756 exciting puzzles for iOS. Collect classic pictures or create puzzles from your own photos. Different difficulty levels, user-friendly interface and saving progress.
Свежее и интересное: https://osago-to.ru/to/forum/messages/forum89/message92002/11186-gde-artodip-gel-kupit-vygodno?result=new#message92002
Стройкаталог https://stroycata1og.ru проекты коттеджей, дома любой площади, каталог стройматериалов. Комплексные услуги от проектирования до сдачи объекта с гарантией качества.
купить бетон бетон купить с доставкой цена
Block Puzzle Wood Classic https://apps.apple.com/ws/app/block-puzzle-wood-classic/id1615792350 is a puzzle game where you need to correctly place wooden blocks. Simple controls, beautiful visuals and addictive gameplay for all ages.
Handmade ceramics flowers Ideal for home, office decor or original gift. Natural beauty and durability.
Бетон в Воронеже https://stk-vrn.ru продажа и доставка. Все марки для фундаментов, дорожных работ и строительства под ключ. Надёжный производитель и лучшие цены.
нтернет-магазин сантехники https://vodomirural.ru ванны, смесители, унитазы, раковины и душевые кабины. Большой выбор, доступные цены, доставка и гарантия качества от производителей.
интернет займ быстрый займ
как взять займ займ онлайн бесплатно
займ оформить микрозайм онлайн
Рейтинг хостингов топ хостингов для сайта подбор сервисов для сайтов и интернет-магазинов. Сравнение тарифов, гарантия стабильности и рекомендации по выбору.
Tickets for Rock Concerts https://rock-concert-tickets.ru
Рестораны Хамовников https://restoran-khamovniki.ru топ заведений для встреч, романтических ужинов и семейных обедов. Авторская кухня, стильный интерьер, удобное расположение и достойный сервис.
Лучшие рестораны https://hamovniki-restoran.ru Хамовников для ценителей гастрономии. Подборка заведений с изысканной кухней, качественным сервисом и атмосферой для отдыха и деловых встреч.
Курсы по плазмотерапии обучение prp освоение методик, современные протоколы, практическая отработка. Обучение для специалистов с выдачей сертификата и повышением квалификации.
Профессиональное обучение онлайн-курсы инъекционной косметологии: подробная программа, практические навыки, сертификация. Освойте эффективные методики для применения в медицине и косметологии.
Детская школа искусств https://elegy-school.ru обучение музыке, танцам, изобразительному и театральному искусству. Творческие программы для детей, концерты, конкурсы и развитие талантов.
Авторские курсы по REVIT https://dashclass.ru обучение созданию интерьеров и архитектурных проектов. Практика, реальные кейсы, индивидуальный подход и профессиональные навыки для работы в проектировании.
Первая помощь детям https://firstaidkids.ru правила оказания при травмах, ожогах, удушье и других ситуациях. Пошаговые инструкции, советы врачей и полезная информация для родителей.
Студия иностранных языков https://whats-up-studiya-inostrannyh-yazykov.ru обучение английскому, немецкому, французскому и другим языкам. Индивидуальные и групповые занятия, современные методики и опытные преподаватели.
палац спорту хмельницький
bazaindex.ru
накрутка подписчиков тг каналов бесплатно без регистрации
Chicken Road gamehttps://apkpure.com/p/app.chickenroad.game
pocket option yasal mı ile güvenle işlem yapmaya başlayın ve sezgisel ve güçlü bir platformdan yararlanın!
удаление катаракты
no deposit casino bonus codes cashable 2021 usa, legitimate online casino in canada and $50 no deposit mobile casino oostende (Beau) new zealand, or online gambling websites
usa
Срочный вызов сантехника https://master-expert.com в Москве на дом. Услуги сантехника: прочистка засоров, ремонт смесителей, установка приборов учета. Работаем 24/7. Недорого и с гарантией. Подробнее на сайте
Детский сад № 55 https://detsadik55.ru забота, развитие и обучение детей. Современные программы, квалифицированные воспитатели, уютные группы, безопасная среда и внимание к каждому ребёнку.
Inizia a fare trading in tutta sicurezza con poket option e goditi una piattaforma intuitiva e potente!
красота beautyhealthclub
Промышленная безопасность https://аттестация-промбезопасность.рф курсы и обучение под ключ. Подготовка к проверке Ростехнадзора, повышение квалификации и сертификация специалистов предприятий.
Автомобили на заказ https://avto-iz-kitaya1.ru поиск, проверка, покупка и доставка. Китай и Корея. Индивидуальный подбор под бюджет и пожелания клиента, полное сопровождение сделки.
Хочу порекомендовать вам телеграм группу для знакомств с девушками в Новосибирске где участницы охотно общаются делятся советами по встречам проводятся тематические мероприятия и есть модерация чтобы новые контакты были безопасными приятными и продуктивными https://t.me/prostitutki_novosibirsk_indi
купить героин траву купить метадон гашиш
ароматические масла aromatmaslo.ru
купить героин спб меф порошок купить
Авто из Китая https://avto-iz-kitaya2.ru на заказ под ключ: подбор, проверка, доставка и растаможка. Новые и подержанные автомобили, выгодные цены и полное сопровождение сделки.
як купити овдп через приват24
https://foodexpert.pro/interesnoe/obuchenie/kak-uchit-angliyskiy-yazyk-detyam-10-13-let-sovety-dlya-roditeley-i-prepodavateley.html
https://kaztur.ru/
ароматы герлен
Авто из Китая под заказ https://dostavka-avto-china.ru кроссоверы, седаны, электромобили и премиальные модели. Индивидуальный подбор, проверка, сопровождение сделки и доставка в ваш город.
Автомобили из Китая на заказ https://avto-iz-kitaya2.ru подбор, покупка и доставка. Полный цикл услуг: диагностика, растаможка, постановка на учёт и гарантия качества.
Funkcje Vavada: Sloty od znanych dostawców Korzystne warunki gry Zróżnicowany program bonusowy Turnieje z dużymi pulami nagród Szybkie transakcje finansowe Profesjonalna obsługa klienta Vavada zdobyła zaufanie i uznanie graczy z wielu krajów świata: Polski, Kazachstanu, Uzbekistanu, krajów europejskich, Azji i Ameryki Południowej.
Эстетическая коррекция рубцов – это сложный процесс, требующий понимания механизмов формирования каждого типа шрама: препараты для инъекционной мезотерапии
духи с сандалом
PuzzleFree online puzzles https://insiderpaper.com/space-tourism-is-quietly-becoming-a-billion-dollar-industry/ hundreds of pictures to assemble, different difficulty levels and user-friendly interface. Enjoy the process, train your memory and attention for free.
Заказать авто из Китая https://dostavka-avto-china2.ru новые автомобили с гарантией, выгодные цены и проверенные поставщики. Доставка, таможня и оформление всех документов под ключ.
https://armada96.ru/
Авто на заказ https://dostavka-avto-russia.ru поиск, диагностика и сопровождение сделки. Машины из Европы, Кореи, Китая и США. Доставка, растаможка и постановка на учёт.
Автомобили на заказ https://dostavka-avto-russia5.ru профессиональный подбор, юридическая проверка, доставка и растаможка. Индивидуальные решения для каждого клиента.
Хотите заказать авто https://prignat-avto5.ru Мы подберём оптимальный вариант, проверим машину по базам, организуем доставку и таможенное оформление. Выгодные цены и прозрачные условия.
купить гранулятор для полимеров производители [url=www.granulyatory-1.ru]купить гранулятор для полимеров производители[/url] .
uralkomenergo.ru [url=https://kompressornyj-zavod-1.ru/]https://kompressornyj-zavod-1.ru/[/url] .
Машины на заказ https://prignat-avto7.ru поиск, диагностика и доставка автомобилей. Индивидуальный подбор, проверенные поставщики и прозрачные условия покупки.
Машины на заказ https://prignat-mashinu5.ru поиск, диагностика и доставка автомобилей. Индивидуальный подбор, проверенные поставщики и прозрачные условия покупки.
Автомобили на заказ https://prignat-mashinu7.ru подбор, проверка, доставка и оформление документов. Машины любых марок и комплектаций с гарантией качества и выгодной ценой.
https://www.legenda.one/
https://widget-free-2x.org/
bouka spins no deposit bonus code, casino coquitlam bc united kingdom and
bet uk things to do Morongo casino, or new casino slot sites usa
Наружная реклама https://pioner-reklama.ru и вывески под ключ: дизайн, производство и монтаж. Световые короба, объёмные буквы, баннеры и витрины. Индивидуальные решения для бизнеса любого масштаба.
Цены на ремонт https://remontkomand.kz/ru/price квартир и помещений в Алматы под ключ. Узнайте точные расценки на все виды работ — от демонтажа до чистовой отделки. Посчитайте стоимость своего ремонта заранее и убедитесь в нашей прозрачности. Никаких «сюрпризов» в итоговой смете!
Ремонт квартир https://remontkomand.kz и домов под ключ: дизайн-проект, отделка, инженерные работы. Работаем по договору, фиксированные сроки и цены. Гарантия качества и полный контроль этапов.
SEO-продвижение сайтов https://raskrutka-sajtov-bystro77.ru в Москве: вывод в ТОП поисковиков, рост трафика и заявок. Полный комплекс — аудит, семантика, оптимизация, ссылки. Эффективное продвижение под ключ.
Планируете ремонт https://remontkomand.kz в Алматы и боитесь скрытых платежей? Опубликовали полный и честный прайс-лист! Узнайте точные расценки на все виды работ — от демонтажа до чистовой отделки. Посчитайте стоимость своего ремонта заранее и убедитесь в нашей прозрачности. Никаких «сюрпризов» в итоговой смете!
https://t.me/ruscasino_top
https://bisant.ru/
https://minprom-sakha.ru/reestr-minpromtorg.html
Планируете ремонт https://remontkomand.kz в Алматы и боитесь скрытых платежей? Опубликовали полный и честный прайс-лист! Узнайте точные расценки на все виды работ — от демонтажа до чистовой отделки. Посчитайте стоимость своего ремонта заранее и убедитесь в нашей прозрачности. Никаких «сюрпризов» в итоговой смете!
Онлайн-курсы шервуд курсы. Изучайте языки, IT, дизайн, маркетинг и другие направления. Удобный формат, доступ к материалам 24/7
https://aqua-basis.ru/
Строительство домов https://stroycata1og.ru и коттеджей под ключ. Готовые проекты, индивидуальные решения, качественные материалы и полный цикл работ — от фундамента до отделки.
Журнал о саде https://nasha-gryadka.ru и огороде онлайн — статьи о выращивании овощей, цветов и фруктов. Советы по уходу, борьбе с вредителями, подбору семян и организации участка.
получить займ онлайн займы деньги
Нужна качественная регулировка окон пвх? Специалисты настроят створки, фурнитуру и уплотнители. Устранение продувания, перекоса и тяжёлого открывания с гарантией качества.
Российская компания «ЗарядЪ» https://заряд.рус поставщик промышленных щелочных и свинцово – кислотных аккумуляторных батарей для резервного электропитания оборудования в разных отраслях . Продукция завода имеет заключение министерства промышленности и торговли Российской Федерации о подтверждении производства промышленной продукции на территории РФ.
Tree removal and care https://www.highqualitytreeservice.com/ pruning, crown shaping, treatment, removal and felling of hazardous trees. Experienced specialists and modern equipment. Safe, professional and with a guarantee of results.
Нужен клининг? лучшие клининговые компании в москве 2026 год. Лучшие сервисы уборки квартир, домов и офисов. Сравнение услуг, цен и отзывов, чтобы выбрать надежного подрядчика.
https://www.legenda.one/
Нужен клининг? рейтинг клининговых компаний москвы 2026. Лучшие сервисы уборки квартир, домов и офисов. Сравнение услуг, цен и отзывов, чтобы выбрать надежного подрядчика.
покупка подписчиков в Телеграм
Стоматологическая клиника https://almazdental35.ru с индивидуальным подходом. Безболезненное лечение, эстетическая стоматология, имплантация и профессиональная гигиена полости рта.
У детей пробелмы с зубами? консультация детского ортодонта — лечение и профилактика зубов у детей. Безопасные методы, комфортная атмосфера и заботливые врачи для маленьких пациентов.
Смотрите сериалы Лучшие сериалы онлайн: https://kineshemec.ru/news/obshhestvo-zhizn/serialy-2025-goda-dla-vechera-doma-smotret-onlajn-v-hd-kachestve-51046.html онлайн в хорошем качестве. Новинки, классика и популярные проекты в удобном формате. Бесплатный просмотр без скачивания и доступ 24/7.
I do not even know how I ended up here, but I thought this post was good. I do not know who you are but certainly you are going to a famous blogger if you are not already 😉 Cheers!
Российская компания «ЗарядЪ» https://заряд.рус поставщик промышленных щелочных и свинцово – кислотных аккумуляторных батарей для резервного электропитания оборудования в разных отраслях . Продукция завода имеет заключение министерства промышленности и торговли Российской Федерации о подтверждении производства промышленной продукции на территории РФ
Нужна контекстная реклама? https://reklama-stomatolog-kontekst.ru под ключ. Настройка в Яндекс.Директ и Google Ads, оптимизация объявлений и контроль заявок.
Лыжи в аренду для взрослых и детей с доставкой на курорт: прокат сноубордов в красной поляне
Комплексные IT-решения https://eclat.moscow для бизнеса: разработка, внедрение, сопровождение и поддержка. Надежные технологии для автоматизации и цифровой трансформации.
СОЛАРТЕК Москва
Play puzzles online https://www.thecoffeemom.net/jigsaw-puzzles-for-moms/ free and without restrictions. A huge selection of pictures, simple controls and the ability to develop attention, memory and thinking. A great way to relax and spend time usefully.
888starz download https://www.pgyer.com/apk/apk/app.starz.online
888starz iOS https://www.pgyer.com/apk/en/apk/app.starz.online
888 starz code bonus https://www.pgyer.com/apk/fr/apk/app.starz.online
swot анализ swot анализ рынка
Took me time to read the material, but I truly loved the article. It turned out to be very useful to me.
https://bar-vip.ru/vyezdnoi_bar/
Большой новогодний шар и новогодние игрушки в Туле. Рекламные агентства сувенирной продукции и корпоративная одежда на заказ с логотипом в Улан-Удэ. Новогодние игрушки шары и подарки на новый год недорогие коллегам в Воронеже. Подарок на день рождения девочке 10 и практичные подарки для женщин в Сочи. Поставщики новогодних игрушек и уличные новогодние оптом елки: сувениров
цель swot анализа стратегия swot анализ
Looking for second-hand? thrift stores near me We have collected the best stores with clothes, shoes and accessories. Large selection, unique finds, brands at low prices. Convenient catalog and up-to-date contacts.
С получением профессиональной помощи в решении юридических вопросов вы можете обратиться к [url=https://konsultaciya-advokata81.ru]юрист консультант онлайн бесплатно без регистрации и[/url], где можно получить юридическую консультацию круглосуточно и бесплатно.
Адвокат окажет необходимую помощь в понимании сложных правовых вопросов.
Looking for second-hand? thrift store near me We have collected the best stores with clothes, shoes and accessories. Large selection, unique finds, brands at low prices. Convenient catalog and up-to-date contacts.
https://1001svet.ru/
ЭВЛО https://evlo-phlebology.ru эндовазальная лазерная коагуляция вен, наряду со склеротерапией является эффективным методом лечения варикозного расширения вен на ногах.
Услуги госпитализации https://hospitall.ru экстренная помощь при угрожающих состояниях и плановое лечение с заранее согласованной программой. Подбор клиники, транспортировка и поддержка пациента на всех этапах.
young escorts – https://moscowescortclub.com/young-escorts/
Great resources and tips for families here.
доставка китай россия доставка карго из китая
фермерские продукты фермерские продукты на дом москва
Онлайн-библиотека Казахстана https://mylibrary.kz книги, статьи, диссертации и редкие издания в цифровом формате. Удобный каталог, быстрый поиск и круглосуточный доступ для всех пользователей.
Эндовазальная лазерная коагуляция https://evlo-phlebology.ru эффективный метод лечения варикоза. Амбулаторная процедура занимает до 40 минут, не требует госпитализации и обеспечивает быстрый косметический и медицинский результат.
Сюрвей грузов сюрвей контроль качества и количества, проверка условий перевозки, составление отчётов. Опытные эксперты обеспечивают объективную оценку для компаний и страховых организаций.
Надежная доставка из китая в Россию: от документов до контейнеров. Выбираем оптимальный маршрут, оформляем таможню, страхуем грузы. Контроль сроков и сохранность на каждом этапе.
tilda разработка сайтов студия веб-дизайна art-made
Надежная доставка грузов из китая: от небольших партий до контейнеров. Авиа, морем, авто и железной дорогой. Оформление, страхование и логистика в кратчайшие сроки.
Нужен автобусный билет? заказ билетов на автобус онлайн просто и удобно. Поиск рейсов, сравнение цен, выбор мест и моментальная оплата. Актуальное расписание, надежные перевозчики и выгодные тарифы каждый день.
Качественный ремонт квартир https://expertremonta.kz от Компании «Эксперт ремонта» это качественный ремонт квартир под ключ в Алматы. Выбирайте Эксперт Ремонта — тут бесплатный выезд замерщика, официальные документы, гарантия документально, ремонт квартир без предоплаты.
доставка диз топлива купить дизельное топливо с доставкой
купить грунт для дачи грунт растительный цена за куб с доставкой
Мобильный выездной шиномонтаж https://master-shin.by круглосуточная помощь в дороге. Экстренная помощь в дороге может понадобиться каждому автолюбителю, и наша компания оказывает ее на высочайшем профессиональном уровне, на самых выгодных в Минске условиях. Оперативный выезд по городу и области, доступные и полностью адекватные цены, квалифицированная сервисная и консультационная поддержка, круглосуточное реагирование, вне зависимости от погодных условий.
Ремонт ноутбуков https://01km.ru телефонов, телевизоров, принтеров и компьютеров в Одинцово и Москве.
go to the website online: https://jnp.chitkara.edu.in
https://ufa.bfm.ru/news/66001
the best site for you: https://telrad.com
visit our website: https://www.acuam.com
our site is waiting for you: https://hyundaimobil.co.id
go to our website: https://petitedanse.com.br
посетить сайт онлайн: https://wildlife.by
our official website: https://fgvjr.com
visit our new website: https://primorskival.si
our new official website: https://www.amakmeble.pl
I found a cool site: https://www.locafilm.com
русское порно инцест https://russkoe-porno1.ru
Want to have fun? porno bangladesh melbet Watch porn, buy heroin or ecstasy. Pick up whores or buy marijuana. Come in, we’re waiting
Mochten Sie ein haus kaufen Montenegro kaufen? Tolle Angebote am Meer und in den Bergen. Gro?e Auswahl an Immobilien, Unterstutzung bei der Immobilienauswahl, Transaktionsunterstutzung und Registrierung. Leben Sie in einem Land mit mildem Klima und wunderschoner Natur.
Новые актуальные промокод iherb promo для выгодных покупок! Скидки на витамины, БАДы, косметику и товары для здоровья. Экономьте до 30% на заказах, используйте проверенные купоны и наслаждайтесь выгодным шопингом.
best articles on the net: https://dnscompetition.in/articles/using-dns-for-secure-remote-access-solutions/
play puzzles online: https://www.reddit.com/r/onlinepuzzlelovers/
Самое лучшее в сети: https://poloniya.ru
best site come on in: https://labhgroup.com
The most useful information: https://satapornbooks.com
Current and up-to-date information: https://clubcoma.org
https://perevod-sochi.com/
доставка из китая карго карго грузы из китая
шпонированные панели дсп стеновые панели мдф
геодезия заказать проведение геодезических изысканий
геотекстиль ландшафтный геотекстиль геоспан
It’s clear you’re passionate about the issues.
Включение в реестр Минпромторга https://minprom-info.ru официальный путь для подтверждения отечественного производства. Подготовка и подача документов, юридическое сопровождение и консультации для производителей.
Занятия по самообороне https://safety-skills.ru практические навыки защиты в реальных ситуациях, развитие силы и выносливости. Профессиональные тренеры помогут освоить приемы борьбы, удары и тактику безопасности.
Платформа онлайн-обучения https://craftsmm.ru курсы по маркетингу, продажам и рекламе для новичков и профессионалов. Освойте современные инструменты продвижения, увеличьте продажи и развивайте карьеру в удобном формате.
rooster bet https://www.pgyer.com/apk/apk/rooster.bet.app
Написание дипломов на заказ https://vasdiplom.ru помощь студентам в подготовке итоговых работ. Авторские тексты, проверка на уникальность и полное соответствие стандартам учебных заведений.
handicap bei basketball punkte wetten
visit our best site online: https://www.maxwaugh.com
The best we have here: https://cour-interieure.fr
http://110km.ru/
NeoSpin Casino app download https://www.pgyer.com/apk/apk/neospin.casino.slots
The latest information is here: https://www.intercultural.org.au
We have the latest information: https://anthese.fr
visit our website: https://cere-india.org
We are waiting for you on the site: https://travel2mv.com
The best of our site: https://www.feldbahn-ffm.de
New and relevant information: https://manorhousedentalpractice.co.uk
Want to have fun? porno girl Whores, drugs, casino. We have it all, any drugs are on sale.
купить меф астана купить трубку для мефа
https://autozames.ru/
Обучение и семинары https://uofs-beslan.ru для профессионалов: современные программы, практические кейсы и опыт экспертов. Развивайте навыки, повышайте квалификацию и получайте новые возможности для карьерного роста.
Академия парикмахерского искусства https://charm-academy.ru обучение от ведущих мастеров. Современные техники стрижек, окрашивания и укладок. Курсы для начинающих и профессионалов с практикой и дипломом по окончании.
Школа видеорекламы https://tatyanamostseeva.ru обучение созданию креативных роликов для бизнеса и брендов. Практические занятия, работа с современными инструментами и поддержка экспертов. Освойте профессию в сфере digital.
Лицей взаимного обучения https://talgenisty.ru уникальная среда для детей и взрослых. Совместные уроки, обмен опытом, мастер-классы и творческие проекты. Образование, основанное на поддержке и сотрудничестве.
The best site for you: https://www.jec.qa
Great site for everyone: https://www.europneus.es
Приём макулатуры https://chsreda.ru в Казани работает как для частных лиц, так и для организаций. Сдают газеты, журналы, картонные коробки, офисную бумагу. Это реально удобно, потому что приезжают, забирают прямо с территории, а потом всё идёт на переработку.
Кухонные принадлежности https://www.kitchen-store.ru/blog/vybrat-bokaly в магазине для дома: современная посуда, полезные гаджеты, текстиль и аксессуары. Стильные решения для кухни, выгодные цены и большой ассортимент.
Нужен автобусный билет? probilets.com удобный сервис поиска и бронирования. Широкий выбор направлений, надежные перевозчики, доступные цены и моментальная отправка электронных билетов на почту.
Авто портал https://diesel.kyiv.ua все о мире автомобилей: новости, обзоры моделей, тест-драйвы, советы по выбору и уходу за авто. Каталог машин, актуальные цены, автоуслуги и полезная информация для автовладельцев.
Автомобильный портал https://auto-club.pl.ua онлайн-площадка для автолюбителей. Подробные обзоры машин, тест-драйвы, свежие новости, советы по ремонту и обслуживанию. Удобный поиск и актуальные материалы.
Все для автомобилистов https://k-moto.com.ua на авто портале: новости, обзоры, статьи, каталоги и цены на автомобили. Экспертные мнения, тест-драйвы и практические советы по эксплуатации авто.
кондиционер для дома [url=https://kondicioner-obninsk-1.ru/]кондиционер для дома[/url] .
монтаж натяжных потолков [url=http://www.natyazhnye-potolki-lipeck-1.ru]http://www.natyazhnye-potolki-lipeck-1.ru[/url] .
sportwetten lizenz curacao
Here is my blog post: darts wettanbieter (dartswettquoten.com)
Нужна виза? открыть визу в италию в москве Консультации, подготовка документов, сопровождение на всех этапах. Визы в Европу, США, Азию и другие страны. Доступные цены и надежная поддержка.
Онлайн женский портал https://elegance.kyiv.ua актуальные советы по красоте, стилю, кулинарии и семейной жизни. Разделы о здоровье, карьере и саморазвитии. Интересные статьи и общение с единомышленницами.
Женский портал https://beautyadvice.kyiv.ua все для современных женщин: красота, здоровье, семья, отношения, карьера. Полезные статьи, советы экспертов, лайфхаки и вдохновение каждый день. Онлайн-сообщество для общения и развития.
Портал для женщин https://fashionadvice.kyiv.ua сайт для девушек и женщин, которые ценят красоту, уют и гармонию. Советы по стилю, отношениям, материнству и здоровью. Читайте статьи, делитесь опытом и вдохновляйтесь новыми идеями.
Авто портал https://avtoshans.in.ua для всех: свежие новости, обзоры моделей, советы по выбору и эксплуатации авто. Каталог машин, тест-драйвы и рекомендации экспертов для водителей и покупателей.
Портал про автомобили https://myauto.kyiv.ua онлайн-ресурс для автолюбителей. Обзоры, статьи, тест-драйвы, цены и полезные советы по ремонту и уходу за машиной. Всё о мире авто в одном месте.
Автомобильные новости https://reuth911.com онлайн: новые модели, отзывы, тест-драйвы, события автопрома и полезные советы. Узнайте первыми о главных новинках и трендах автомобильного мира.
Свежие новости авто https://orion-auto.com.ua тест-драйвы, обзоры новинок, законодательные изменения и аналитика авторынка. Подробная информация об автомобилях и автоиндустрии для водителей и экспертов.
Автомобильный сайтhttps://setbook.com.ua свежие новости, обзоры моделей, тест-драйвы и советы экспертов. Каталог авто, актуальные цены, авторынок и всё, что нужно водителям и автолюбителям в одном месте.
Онлайн-сайт для женщин https://musicbit.com.ua стиль, уход за собой, психология, семья, карьера и хобби. Интересные статьи, тесты и форум для общения. Пространство для вдохновения и развития.
Онлайн-журнал для женщин https://fines.com.ua стиль, уход за собой, психология, рецепты, материнство и карьера. Актуальные материалы, тренды и экспертные рекомендации каждый день.
Женский сайт о жизни https://prettywoman.kyiv.ua секреты красоты, мода, здоровье, рецепты и отношения. Интересные статьи, советы и лайфхаки. Всё, что нужно, чтобы чувствовать себя уверенно и счастливо.
Some truly interesting info , well written and broadly user genial .
Онлайн-сайт про автомобили https://tvregion.com.ua свежие новости, аналитика рынка, обзоры и сравнения машин. Советы по обслуживанию и выбору авто. Всё для водителей и автолюбителей в одном месте.
Женский онлайн-журнал https://feminine.kyiv.ua мода, красота, здоровье, отношения и семья. Полезные советы, вдохновляющие статьи, лайфхаки для дома и карьеры. Всё самое интересное для современных женщин.
Автомобильный портал https://troeshka.com.ua онлайн-ресурс для автовладельцев. Каталог машин, тест-драйвы, аналитика авторынка и советы специалистов. Будьте в курсе новинок и технологий автоиндустрии.
Сайт для женщин https://lolitaquieretemucho.com мода, красота, здоровье, отношения, семья и карьера. Полезные советы, статьи, рецепты и лайфхаки. Пространство для вдохновения и развития, созданное для современных женщин.
Сайт для женщин https://femaleguide.kyiv.ua гармония стиля и жизни. Уход за собой, рецепты, дом, отношения, карьера и путешествия. Читайте статьи, делитесь опытом и вдохновляйтесь новыми идеями.
Автомобильный новостной портал https://tuning-kh.com.ua всё об авто в одном месте: новости, цены, обзоры, тест-драйвы, авторынок. Советы экспертов и полезные материалы для водителей и тех, кто планирует купить машину.
Сайт про машины https://tvk-avto.com.ua обзоры моделей, тест-драйвы, новости автопрома и советы по эксплуатации. Полезные статьи о выборе авто, уходе, ремонте и актуальные материалы для автовладельцев.
Женский онлайн портал https://femalesecret.kyiv.ua онлайн-ресурс для девушек и женщин. Мода, красота, здоровье, семья и материнство. Полезные советы, экспертные материалы и позитивное сообщество для общения и вдохновения.
besten wett tipps
my site … sportwetten forum strategie – Domenic –
Онлайн-сайт для женщин https://mirlady.kyiv.ua красота, стиль, здоровье, дом и семья. Практичные рекомендации, модные идеи, вдохновение и поддержка. Лучший контент для девушек и женщин любого возраста.
Сайт для женщин https://amideya.com.ua портал о красоте, стиле, здоровье, семье и саморазвитии. Ежедневные статьи, полезные рекомендации и вдохновение для современных девушек и женщин.
Женский сайт https://lubimoy.com.ua стиль, уход за собой, психология, материнство, работа и хобби. Актуальные статьи, тренды и экспертные советы. Всё самое важное для гармоничной жизни и успеха.
Женский онлайн-журнал https://gracefullady.kyiv.ua свежие статьи о моде, красоте, здоровье и саморазвитии. Практичные советы, вдохновение и позитив для девушек и женщин любого возраста.
latest information here: https://astra-hotel.ch
Here is the best on the net: https://www.manaolahawaii.com
visit our website: https://lmc896.org
Latest information from us: https://www.europneus.es
The latest data is right here: https://m120.com
Go to our website: http://redeecologicario.org
All the latest on our website: https://clubcoma.org
Here you will find only the best: https://jonathanlittlepoker.com
I am glad to be a visitor of this thoroughgoing web blog ! , regards for this rare information! .
Come in – don’t miss the latest: https://www.manaolahawaii.com
Only we have the newest materials: https://justicelanow.org
Always relevant information: https://agriness.com
Information that is always relevant: https://billi-walker.jp
Always relevant here: https://penzo.cz
Catch the latest from us: https://eguidemagazine.com
Everything new is nearby: https://jnp.chitkara.edu.in
Сайт finance-post.ru — это блог финансиста, ориентированный на личные финансы, бюджетирование и инвестиции. На нём публикуются практические материалы, советы и обзоры
neue wettanbieter deutschland
Feel free to surf to my web blog :: Kombiwette erklärung
Печатный Центр https://kopirych.by «Копирыч» в Минске и по всей Беларуси! Оперативная печать, брошюровка, широкоформатная и сувенирная продукция. Качественно, недорого, с доставкой. Ваши идеи — наша реализация! Заказывайте на сайте или по телефону.
руководства по садоводству https://zelenayazona.ru идеи, инструкции и советы по уходу за садом и огородом. Информация для новичков и опытных дачников: посадка, полив, удобрение и защита растений.
Женский сайт https://family-site.com.ua современный портал о моде, красоте, отношениях и саморазвитии. Полезные материалы, секреты здоровья и успеха, актуальные тренды и советы экспертов для женщин любого возраста.
Семейный портал https://geog.org.ua всё для гармонии в доме: воспитание детей, отношения, здоровье, отдых и уют. Полезные советы, статьи и лайфхаки для всей семьи. Пространство, где находят ответы и вдохновение.
Современный женский https://happywoman.kyiv.ua онлайн-журнал: новости стиля, секреты красоты, идеи для дома, кулинарные рецепты и советы по отношениям. Пространство для вдохновения и развития.
Женский онлайн портал https://femalesecret.kyiv.ua онлайн-ресурс для девушек и женщин. Мода, красота, здоровье, семья и материнство. Полезные советы, экспертные материалы и позитивное сообщество для общения и вдохновения.
скачать приложение 888starz https://corporaciondelsur.com.py/2025/09/08/skachat-888starz-nate-android-bezvozmezdno-dolzhnostnaya-mobilnaya-variant-vozmite-russkom/
Современный женский https://happywoman.kyiv.ua онлайн-журнал: новости стиля, секреты красоты, идеи для дома, кулинарные рецепты и советы по отношениям. Пространство для вдохновения и развития.
Портал о стройке https://bastet.com.ua статьи, новости и советы по ремонту, строительству и дизайну. Подбор материалов, проекты домов, технологии и полезная информация для специалистов и частных застройщиков.
Портал о здоровье https://mikstur.com информационный ресурс о медицине и ЗОЖ. Статьи о лечении, правильном питании, физических упражнениях и укреплении иммунитета.
Информационный портал https://intertools.com.ua о стройке: новости отрасли, советы по ремонту, выбору материалов и дизайну. Всё для тех, кто строит дом, делает ремонт или работает в строительстве.
Портал про детей https://mch.com.ua информационный ресурс для родителей. От беременности и ухода за малышом до воспитания школьников. Советы, статьи и поддержка для гармоничного развития ребёнка.
Женский онлайн-журнал https://girl.kyiv.ua стиль, уход за собой, психология, кулинария, отношения и материнство. Ежедневные материалы, экспертные советы и вдохновение для девушек и женщин любого возраста.
Онлайн-журнал для женщин https://krasotka-fl.com.ua всё о красоте, моде, семье и жизни. Полезные статьи, лайфхаки, советы экспертов и интересные истории. Читайте и вдохновляйтесь каждый день.
I wanted to check up and let you know how, a great deal I cherished discovering your blog today. I might consider it an honor to work at my office and be able to utilize the tips provided on your blog and also be a part of visitors’ reviews like this. Should a position associated with guest writer become on offer at your end, make sure you let me know.
Our family had similar issues, thanks.
Онлайн-журнал https://presslook.com.ua для женщин объединяет всё, что важно: мода и стиль, воспитание детей, карьерные советы и вдохновение. Советы специалистов и реальные истории для поддержки и новых идей.
Актуальные тренды https://horoscope-web.com и вневременная классика. Подборки образов, советы по стилю, секреты гардероба и модные инсайты. Мы поможем тебе выглядеть безупречно каждый день и выразить свой индивидуальный стиль.
Твой гид https://nicegirl.kyiv.ua по здоровому образу жизни! Эффективные тренировки, сбалансированное питание, wellness-практики и советы по мотивации. Обрети энергию, силу и гармонию в теле, которое ты любишь.
Ресурс для амбициозных https://ramledlightings.com и целеустремленных. Карьерный рост, личная эффективность, финансовая грамотность и вдохновляющие истории успеха. Реализуй свой потенциал и добивайся всех поставленных целей!
These are some of the most important issues we’ll face over the next few decades.
выполнение курсовых стоимость написания курсовой
оформить займ онлайн взять займы онлайн без карты
взять онлайн займ на карту займы онлайн без отказа с плохой
Puzzles online https://github.com/ivanjarkov play for free in assembling pictures of any complexity. Thousands of options: classic, children’s, 3D and thematic. Convenient interface, saving progress and new puzzles every day.
888starz https://www.porta.com.tr/888starz-888starz-ofitsialnyy-veb-zhurnal-igornyy-dom-a-takzhe-bk-2025/
bonus code sportwetten
Also visit my website; unentschieden wette ungültig kombiwette
скачать приложение 888starz https://blockchaininvestmentcouncil.com/blog/bukmekerskaya-administratsiya-888starz-veb-obozrenie-bonusy-koeffitsienty
888starz скачать на айфон https://culturadom.fr/2025/09/03/888starz-oformlenie-a-eshche-verbovoe-vozmite-dolzhnostnoy-zhurnal-igornyy-dom-888-starz-gde-segodnya-nuzhno-zakachivat-i-tantsevat/
Твой гид https://womanlife.kyiv.ua по стильной жизни. Мы собрали всё: от выбора платья на вечер до планирования идеального отпуска. Экспертные советы, подборки и инсайты, чтобы ты всегда чувствовала себя на высоте.
Журнал для женщин https://rpl.net.ua которые строят карьеру и хотят большего. Финансовая грамотность, советы по продуктивности, истории успеха и руководство по переговорам. Достигайте своих целей с нами!
Онлайн-журнал о моде https://glamour.kyiv.ua без правил. Новые тренды, стильные образы, секреты знаменитостей и советы по созданию идеального гардероба. Мы поможем вам найти и с уверенностью выразить свой уникальный стиль.
Женский сайт https://bbb.dp.ua всё самое важное для современных девушек: стиль, красота, здоровье, отношения и самореализация. Читайте, вдохновляйтесь и находите новые идеи.
Новостной портал Украины https://lenta.kyiv.ua оперативные события в стране. Политика, экономика, региональные новости, спорт и культура. Достоверные материалы и аналитика каждый день.
Новостной сайт https://vesti.in.ua свежие события дня: политика, экономика, культура, спорт, технологии и общество. Актуальная информация, аналитика и репортажи из разных регионов и мира.
Свежие новости https://sensus.org.ua Украины и мира: главные события, репортажи и аналитика. Политика, экономика, общество и культура в удобном формате онлайн.
Свежие новости Украины https://novosti24.kyiv.ua главные события, мнения экспертов и аналитические материалы. Лента новостей онлайн, репортажи и достоверные факты без перерыва.
888 starz casino https://cursos.institutofernandabenead.com.br/888starz-igornyy-dom-i-professiya-4000-igr-i-pribylnye-stavki/
888starz bet скачать http://en.speakee.fr/rabochee-luchnik-888starz-casino-a-eshche-ofitsialnyy-zhurnal/
back lay wetten deutschland (Valeria) erklärung
Новости Украины https://status.net.ua объективная информация о событиях страны. Политика, экономика, региональные новости, спорт и культура. Читайте актуальные материалы каждый день.
Новостной портал https://mediateam.com.ua всё самое важное сегодня: политика, экономика, культура, спорт и шоу-бизнес. Лента новостей, репортажи и аналитические материалы каждый день.
Новости Украины и мира https://mostmedia.com.ua политика, экономика, культура, спорт и общество. Свежие события, аналитика и репортажи. Будьте в курсе главных новостей в режиме онлайн 24/7.
Необходимо кодирование? кодировка от алкоголя в Хабаровске современные методы, конфиденциальность и поддержка специалистов. Помогаем избавиться от зависимости и вернуться к здоровой жизни.
wettbüro
Review my web blog: wettstrategien Sportwetten
Аренда авто https://myrentacar.site широкий выбор автомобилей для любых целей. Удобное бронирование, доступные цены, страхование и гибкие условия. Машины в отличном состоянии для поездок по городу и путешествий.
Строительство домов в Сочи https://stroitelstvodomovsochi.ru под ключ — профессиональный подход, проверенные материалы и гарантия качества. Полный спектр работ: проект, строительство, отделка и благоустройство.
нарколог вывод из запоя прокапаться от алкоголя
Онлайн новостной портал https://reporternews.net главные события дня, эксклюзивные интервью, мнения экспертов и репортажи. Достоверная информация о политике, бизнесе и жизни общества.
Новостной портал https://newsawait.com свежие новости, аналитика и обзоры. Политика, экономика, культура и спорт. Лента событий в режиме реального времени с проверенными фактами.
Портал про авто https://dream-autos.com новости, обзоры и тест-драйвы. Полезные советы по выбору, ремонту и эксплуатации автомобилей. Каталог машин, актуальные цены и аналитика авторынка.
mm88 là sân chơi cá cược đẳng cấp hàng đầu Châu Á. Sở hữu kho game trực tuyến đa dạng từ: xổ số, casino, thể thao,… Tải App để tham gia trải nghiệm mượt …
Новости Украины и мира https://globalnewshome.com всё самое важное сегодня. Политика, экономика, региональные события, спорт и культура. Объективные статьи и аналитика в удобном формате.
Портал для женщин https://womanfashionista.com всё самое важное в одном месте: уход за собой, мода, дом, семья и карьера. Читайте полезные статьи, находите вдохновение и делитесь опытом.
Сайт детского сада https://malush16.ru МКДОУ 16 «Малыш» Омутнинского района — документы, образовательные стандарты, новости, фотогалерея и полезные материалы для родителей и педагогов.
Статьи для садоводов https://portalteplic.ru огородников, фермеров и пчеловодов: советы по уходу за растениями, животными и пасекой. Полезные инструкции, лайфхаки и сезонные рекомендации.
Портал о ремонте https://studio-nd.ru статьи, инструкции и советы для дома и квартиры. От выбора материалов до дизайна интерьеров. Полезные рекомендации для мастеров, новичков и частных застройщиков.
Сайт про металлопрокат https://the-master.ru каталог продукции, характеристики и сферы применения. Арматура, балки, трубы, листы и профили. Актуальные цены, советы специалистов и полезные статьи.
Строительный портал https://krovlyaikrysha.ru база знаний и идей. Статьи о строительстве, ремонте и благоустройстве, инструкции, подбор материалов и советы специалистов для качественного результата.
Всё про ремонт https://gbu-so-svo.ru и строительство — статьи, инструкции и советы для мастеров и новичков. Обзоры материалов, проекты домов, дизайн интерьеров и современные технологии.
Автомобильный портал https://ivanmotors.ru всё о машинах в одном месте. Тест-драйвы, обзоры, аналитика авторынка и советы специалистов. Актуальные события мира авто для водителей и экспертов.
888starz зеркало рабочее на сегодня https://centralpaulista.com.br/sem-categoria/888starz-bukmekerskaya-kontora-stavki-na-aviasport/
Сайт о ремонте https://e-proficom.ru полезные статьи, пошаговые инструкции и советы экспертов. От выбора материалов до дизайна интерьеров. Всё, что нужно для ремонта квартир и домов.
Сайт для женщин https://devchenky.ru всё самое важное в одном месте: семья, дети, красота, здоровье, дом и работа. Советы специалистов, лайфхаки и вдохновение на каждый день.
Блог о ремонте https://ivinstrument.ru полезные статьи, пошаговые инструкции и советы экспертов. Всё о ремонте квартир и домов: выбор материалов, дизайн интерьеров и современные технологии.
Современные наливные полы https://proffi-floor.ru это не только идеально ровная поверхность, но и долговечность покрытия. Если вы планируете ремонт в квартире или офисе, узнайте подробнее об услугах по устройству наливных полов
Городской портал Москвы https://moscowfy.ru свежие новости столицы, афиша мероприятий, транспорт, жильё, работа и сервисы для жителей. Полезная информация для москвичей и гостей города на одном сайте.
заверенный перевод документов бюро переводов нотариус
стоматология телефон [url=https://www.stomatologiya-voronezh-1.ru]стоматология телефон[/url] .
wettquote bundestagswahl
Feel free to surf to my page best online sportwetten (Site-dbahtosuv.godaddysites.com)
Подборка статей https://yandex-direct-info.ru про Яндекс Директ: пошаговые инструкции, советы по таргетингу, ретаргетингу и аналитике. Всё о рекламе в Яндексе в одном месте для вашего бизнеса.
Яндекс Бизнес https://business-yandex3.ru описание сервиса, его инструменты и функции. Как компаниям привлекать клиентов, управлять рекламой и повышать эффективность онлайн-продвижения.
Hi, possibly i’m being a little off topic here, but I was browsing your site and it looks stimulating. I’m writing a blog and trying to make it look neat, but everytime I touch it I mess something up. Did you design the blog yourself?
Your resources are well developed.
sichere kombiwetten
Here is my blog über unter wetten erklärung
All the most important here: https://possible11.com
Игра в города https://cl-news.ru/puteshestvie-po-gorodam-na-bukvu-a/ для всех возрастов. Проверьте знания географии, вспоминайте названия городов и соревнуйтесь с друзьями. Увлекательная логическая игра для тренировки памяти и эрудиции.
Open the latest data here: https://www.acuam.com
The most relevant place is here: https://woodbridgebrewingco.com
Details are waiting for you here: https://www.guidasposi.it
Only verified facts are here: https://trakdag.com
Everything in one place: https://clubcoma.org
Read the best here: https://eguidemagazine.com/
Everything you need: https://stovespareparts.ie
News for you – right here: https://fish-pet.com
Hot news is already here: https://2tk.pl
Everything the brightest here: https://bookingautos.com
sportwetten bester anbieter
Here is my web blog :: Buchmacher Us Wahl
Топливные карты https://alcomauto.ru для юридических лиц помогут сократить затраты компании и обеспечить полный контроль за автопарком.
Топливные карты https://karta-vezdehod.ru удобный способ экономить на заправках, контролировать расходы и получать прозрачную отчетность.
Топливные карты https://auto-nv.ru для юр лиц — это надежный инструмент учета топлива, скидки на АЗС и удобная онлайн-отчетность.
sportwetten unentschieden vorhersagen
Also visit my web site – comment-137591
Топливная карта https://autobsn.ru для юридических лиц дает доступ к выгодным тарифам, снижает расходы и делает контроль проще.
Что такое Agile https://agile-metod.ru и как его внедрить? Подробные статьи о гибких методологиях, инструментах и практиках. Scrum, Kanban и Lean — всё о современном управлении проектами.
Что такое CPI https://cost-per-install.ru в маркетинге? Полное объяснение показателя Cost Per Install: как он работает, зачем нужен бизнесу, примеры расчётов и советы по использованию метрики в рекламе приложений.
sportwetten tipps wochenende
Here is my site; Kombiwette Quote berechnen
plug in prague buy xtc prague
online sportwetten ohne lugas
Also visit my site … Internet Wetten
prague plug vhq cocaine in prague
höchster sportwetten bonus
Also visit my web-site WettbüRo Ludwigshafen
Графитовые и угольные щетки для электроинструмента. Большой выбор, надёжность и долговечность. Подходят для дрелей, болгарок, перфораторов и другого оборудования.
Нужны двери? межкомнатные двери интернет магазин спб Широкий ассортимент межкомнатных дверей от Вектордорс. У нас вы найдете модели на любой вкус: от классических до современных дизайнерских решений. Выбор межкомнатных дверей — важный этап обустройства помещения. Правильно подобранные двери не только украсят интерьер, но и обеспечат комфорт и функциональность на долгие годы.
sportwetten quoten heute [Freeicons.io] beste app
bonus martingale strategie Sportwetten vergleich
доставка щебня на объект продажа строительной глины
CPL (Cost Per Lead) https://cost-per-lead1.ru ключевая метрика рекламы. Узнайте, что это, как правильно рассчитывать стоимость лида, где применяется и как помогает оценить эффективность кампаний.
Интернет-маркетинг https://internet-marketing1.ru SEO, контекстная реклама, SMM, email-рассылки и аналитика. Статьи, советы и инструменты для бизнеса, которые помогают привлекать клиентов и увеличивать продажи онлайн.
Hello, I think your blog might be having browser compatibility issues. When I look at your website in Chrome, it looks fine but when opening in Internet Explorer, it has some overlapping. I just wanted to give you a quick heads up! Other than that, awesome blog!
Интернет-маркетинг https://yandex-reklama2.ru для компаний и специалистов: SEO, SMM, контекстная реклама и email. Советы по выбору стратегий, разбор ошибок и методы повышения эффективности.
wettanbieter paypal
Here is my webpage: sportwetten mit Startguthaben ohne einzahlung
wetten italien deutschland
Feel free to surf to my website – comment-69521
Памятники на заказ https://top-memorial.ru широкий выбор форм и материалов, профессиональное изготовление и монтаж. Индивидуальный подход, гарантия качества и доступные цены.
Клининговая компания https://cleaningplus.ru в Москве: профессиональная уборка квартир, домов и офисов. Генеральная, ежедневная, послестроительная уборка, химчистка мебели и ковров. Доступные цены и гарантия качества.
На сайте «Детский Класс» https://www.detskiyklass.ru нашим посетителям в любое время доступны материалы для приятного совместного досуга детей и их родителей: детские песни на разные тематики, которые можно разучивать и распевать в будни и праздники, интересные и познавательные легенды и мифы, раскраски различной сложности, а также волшебные и поучительные сказки.
http://pacireland.eu/?attachment_id=2951#comment-3716
buy cocaine in telegram prague drugs
cocaine prague plug in prague
sportwetten profi strategie
my homepage: comment-99391
You need to really control the comments listed here
cocaine prague telegram cocain in prague from columbia
XOSO66 là nền tảng xổ số trực tuyến quốc tế, ra đời với sứ mệnh mang đến cho người chơi một môi trường giải trí minh bạch, công bằng và hiện đại.
устройство плоской кровли https://ploskaya-krovlya-pod-klyuch.ru
Tips and tools you offer are so helpful to agencies in our community.
I just sent this post to a bunch of my friends as I agree with most of what you’re saying here and the way you’ve presented it is awesome.
Портал про авто https://ivanmotors.ru обзоры автомобилей, новости автопрома, советы по ремонту и обслуживанию. Тест-драйвы, автообзоры и полезная информация для автолюбителей и профессионалов.
Всё о Москве https://moscowfy.ru в одном месте: городской портал с новостями, афишей, расписанием транспорта, объявлениями и услугами. Полезные материалы для москвичей и туристов.
Портал о строительстве https://e-proficom.ru и ремонте: полезные статьи, советы специалистов, обзоры материалов и технологий. Всё для тех, кто планирует ремонт квартиры, дома или дачи.
Женский портал https://devchenky.ru секреты красоты, модные тенденции, здоровье, любовь и кулинария. Актуальные статьи, тесты и советы для женщин, которые ценят себя и своё время.
40 shining crown bell link çox sevilən versiyadır.
Shining crown slot game yüksək keyfiyyətli qrafika ilə hazırlanıb.
Superbet demo shining crown ilə oyun pulsuzdur. Shining crown free play hər kəs üçün əlçatan seçimdir. Shining crown buy bonus seçimi əlavə həyəcan yaradır.
Shining crown pacanele Rumıniya bazarında liderdir.
Shining crown demo superbet sürətli işləyir.
Daha ətraflı buradan baxın [url=https://shining-crown.com.az/]shining crown demo[/url].
40 shining crown klassik və sadə oyun mexanikası təqdim edir.
Shining crown pacanele oyunçular arasında çox populyardır.
Weboldalunk, a joszaki.hu buszken tamogatja a kormanypartot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
Строительные материалы https://stroy-marketplace.ru в Серпухове: кирпич, цемент, сухие смеси, пиломатериалы и утеплители. Большой выбор для ремонта и строительства, доставка по городу и району.
Компания Единство https://edinvent.ru производитель и комплексный поставщик приточно-вытяжных установок, вентиляционных установок, а также систем автоматического управления климатическим оборудованием.
Женский блог https://www.evasolar.ru о здоровье и красоте: советы по уходу за собой, правильному питанию, фитнесу и косметике. Полезные статьи, лайфхаки и вдохновение для гармоничной и счастливой жизни.
Play shining crown oyunu ilə böyük həyəcan yaşamaq mümkündür.
Shining crown joc gratis pulsuz təcrübə üçün əladır.
Shining crown 40 slotları klassik kombinasiya təklif edir. Shining crown casino etibarlı oyun mühitinə zəmanət verir. Pinco casino shining crown üçün ən yaxşı platformadır.
Shining crown pacanele Rumıniya bazarında liderdir.
Shining crown rtp oyunun ədalətini təsdiqləyir.
Casino üçün keçid [url=https://shining-crown.com.az/]online casino link[/url].
40 shining crown klassik və sadə oyun mexanikası təqdim edir.
Shining crown casino hər kəs üçün etibarlı platformadır.
Hello there I am so delighted I found your weblog, I really found you by mistake, while I was searching on Google for something else, Anyhow I am here now and would just like to say cheers for a remarkable post and a all round exciting blog (I also love the theme/design), I don’t have time to browse it all at the moment but I have book-marked it and also included your RSS feeds, so when I have time I will be back to read a lot more, Please do keep up the superb work.
Shining crown demo oyna imkanı yeni başlayanlar üçün faydalıdır.
Shining crown pacanele Rumıniyada məşhurdur, Azərbaycanda da sevilir.
Shining crown bell link demo yeni oyunçular üçün idealdır. Shining crown free play hər kəs üçün əlçatan seçimdir. Shining crown lines oyunçular üçün geniş imkanlar açır.
Shining crown demo oyna pulsuz və sürətlidir.
Shining crown rtp oyunun ədalətini təsdiqləyir.
Domen ünvanı [url=https://shining-crown.com.az/]domen com[/url].
Shining crown big win uduşları oyunçular üçün motivasiya rolunu oynayır.
Superbet demo shining crown təcrübəni pulsuz təqdim edir.
Play shining crown oyunu ilə böyük həyəcan yaşamaq mümkündür.
Shining crown big win qazanmaq motivasiya yaradır.
Shining crown nasıl oynanır sualına cavab çox sadədir. Shining crown amusnet çoxlu oyun variantı ilə tanınır. Shining crown buy bonus seçimi əlavə həyəcan yaradır.
Play shining crown asan interfeys ilə təklif olunur.
Shining crown online sadə və əlçatan platformadır.
Rahat giriş imkanı var [url=https://shining-crown.com.az/]shining crown online[/url].
Shining crown online casino rahat giriş imkanı verir.
Shining crown casino hər kəs üçün etibarlı platformadır.
Cricket betting https://baji-bj.com
Прайс-лист на бетонні роботи перегляньте на remontuem.te.ua
Shining crown demo oyna imkanı yeni başlayanlar üçün faydalıdır.
Crown shining sadə və maraqlı oyun təcrübəsidir.
Shining crown gratis versiyası limitsiz sınaq verir. Shining crown demo ron Avropa bazarında məşhurdur. 40 shining crown bell link ən məşhur slotlardan biridir.
Shining crown joc gratis təcrübə qazandırır.
Shining crown free slot real uduş öncəsi sınamağa imkan verir.
Domen ünvanı [url=https://shining-crown.com.az/]domen com[/url].
Shining crown online casino rahat giriş imkanı verir.
Shining crown lines qazanma yollarını artırır.
Descopera joaca-tancuri.top – simulator de razboi cu tancuri istorice. Lupte dinamice, har?i variate ?i batalii tactice. Creeaza-?i strategia ?i domina impreuna cu prietenii tai.
Joaca World of Warship gratuit! Exploreaza marile, folose?te-?i strategia ?i condu nave de razboi celebre. Batalii realiste ?i echipe interna?ionale te a?teapta.
Descubra o caca-niqueis jogo 9 Masks of Fire – um jogo cheio de adrenalina, simbolos especiais e rodadas bonus. Experimente graficos intensos e altas chances de ganhar em um caca-niqueis classico moderno.
Jogue pands slots e descubra o charme do Oriente! Rolos coloridos, recursos bonus, rodadas gratis e surpresas que trazem ganhos espetaculares.
ваш провідник у житті Львова https://79000.com.ua актуальні новини, культурні та громадські події міста, урбаністика, інтерв’ю з цікавими людьми, фотоогляди локальних заходів. Все про те, що формує атмосферу Львова сьогодні — оновлення, проекти, історії.
інформаційний портал https://21000.com.ua Вінниці і області: місцеві новини, анонси культурних, спортивних та громадських подій, репортажі з місця подій, інтерв’ю з вінничанами. Все про те, що відбувається у Вінниці — ближче, живіше, щодня.
ООО “Мир ремней” https://yandex.ru/profile/147633783627 Производство приводных ремней, тефлоновых сеток и лент – телефон +7 (936) 333-93-03
A cool post there mate ! Thank you for posting.
Пиломатериалы в Минске https://farbwood.by оптом и в розницу. Доска обрезная и строганая, брус, лаги, террасная доска. Качественная древесина для строительства и ремонта. Быстрая доставка.
займ денег оформить займ онлайн
займ деньги взять займ
онлайн займы займ денег онлайн
Нужны автостекла? ремонт автостекол в спб Khan Auto Glass — это профессиональная компания, которая специализируется на продаже и установке автомобильных стёкол в Санкт-Петербурге. Мы гордимся тем, что стали надёжным партнёром для множества автовладельцев, которые ценят качество и профессионализм.
Договоры и доверенности – бюро переводов диплома. Бюро переводов в Самаре для макетов и верстки. Сохранение исходного форматирования в PDF, InDesign. Переводчик и дизайнер в одном лице.
веранда к дому https://remontuem.te.ua
Нужна лабораторная? лабораторные на заказ Индивидуальный подход, проверенные решения, оформление по требованиям. Доступные цены и быстрая помощь.
Нужна презентация? https://prez-shablony-ucheb.ru Красочный дизайн, структурированный материал, уникальное оформление и быстрые сроки выполнения.
Нужен чертеж? https://chertezhi-kurs.ru выполним чертежи для студентов на заказ. Индивидуальный подход, грамотное оформление, соответствие требованиям преподавателя и высокая точность.
Нужна лабораторная? заказы по лабораторной Индивидуальный подход, проверенные решения, оформление по требованиям. Доступные цены и быстрая помощь.
40 shining crown bell link çox sevilən versiyadır.
Shining crown 77777 slot oyunu fərqli simvollarla doludur.
Shining crown slot oyna mobil və PC üçün uyğundur. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. Shining crown apk mobil cihazlarda problemsiz işləyir.
Shining crown demo oyna pulsuz və sürətlidir.
Shining crown buy bonus oyunçulara əlavə qazanc gətirir.
Rəsmi domen buradadır [url=https://shining-crown.com.az/]http://shining-crown.com.az/[/url].
Shining crown slot game yüksək qrafika və səs effektləri ilə zəngindir.
Shining crown pacanele oyunçular arasında çox populyardır.
Нужна презентация? заказать презентацию в повер поинт Красочный дизайн, структурированный материал, уникальное оформление и быстрые сроки выполнения.
Нужен чертеж? где можно заказать чертежи выполним чертежи для студентов на заказ. Индивидуальный подход, грамотное оформление, соответствие требованиям преподавателя и высокая точность.
I have been curious about these trends, and you have really helped me. I have just told a few of my friends about this on FaceBook and they love your content just as much as I do.
cocaine in prague cocain in prague from peru
cocaine prague prague drugstore
Вызвать дерматолога на дом https://www.docplus24.ru
Weboldalunk, a joszaki.hu weboldalunk buszken tamogatja a kormanyzo partot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
Weboldalunk, a joszaki.hu weboldalunk buszken tamogatja a kormanyzo partot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
сантехнік тернопіль ціни https://remontuem.te.ua
Авто в ОАЭ https://auto.ae покупка, продажа и аренда новых и б/у машин. Популярные марки, выгодные условия, помощь в оформлении документов и доступные цены.
займ взять микрозайм получить
Заметки практикующего врача https://phlebolog-blog.ru флеболога. Профессиональное лечение варикоза ног. Склеротерапия, ЭВЛО, УЗИ вен и точная диагностика. Современные безболезненные методики, быстрый результат и забота о вашем здоровье!
purebred kittens for sale in NY https://catsdogs.us
Проблемы с откачкой? насос для откачки воды из подвала сдаем в аренду мотопомпы и вакуумные установки: осушение котлованов, подвалов, септиков. Производительность до 2000 л/мин, шланги O50–100. Быстрый выезд по городу и области, помощь в подборе. Суточные тарифы, скидки на долгий срок.
Нужна презентация? нейросеть сделать презентацию Создавайте убедительные презентации за минуты. Умный генератор формирует структуру, дизайн и иллюстрации из вашего текста. Библиотека шаблонов, фирстиль, графики, экспорт PPTX/PDF, совместная работа и комментарии — всё в одном сервисе.
buy coke in prague cocain in prague from dominican republic
cocaine prague prague drugstore
coke in prague cocaine in prague
Ən yaxşı seçimlərdən biri də Sunny Coin Hold The Spin onlayn versiyasıdır. Sunny Coin 2: hold the spin demo rahat və əlçatandır.
Sunny Coin Hold The Spin slotunu sınamaq xoşdur. Sunny coin hold the spin slot onlayn kazinolarda məşhurdur.
Demo versiya üçün keçid [url=https://sunny-coin.com.az/]http://sunny-coin.com.az[/url].
Sunny coin 2: hold the spin slot pulsuz versiya rahatdır. Sunny coin hold the spin oyna və fərqli təcrübə qazan. Sunny coin hold the spin slot online rahat oynanır.
Sunny coin hold the spin slot dizaynı çox rəngarəngdir. Sunny Coin Hold The Spin real pul üçün əlverişlidir.
pure cocaine in prague high quality cocaine in prague
cocaine prague buy coke in prague
Maraqlı dizayn və yüksək RTP ilə Sunny Coin Hold The Spin fərqlənir. Sunny Coin 2 hold the spin slot real pul ilə daha həyəcanlıdır.
Sunny coin: hold the spin slot RTP yüksək olduğu üçün sevilir. Sunny coin 2 hold the spin slot qazancları maraqlı edir.
Pulsuz demo üçün keçid edin [url=https://sunny-coin.com.az/]sunny coin: hold the spin slot[/url].
Sunny Coin Hold The Spin slot böyük qaliblərə şans verir. Sunny coin hold the spin slot bonus raundları çox cəlbedicidir. Sunny coin: hold the spin slot RTP səviyyəsi yüksəkdir.
Sunny Coin Hold The Spin slot online hər kəs üçün əlçatan olur. Sunny coin: hold the spin slot təcrübəsi həyəcanlıdır.
Sunny coin 2: hold the spin ilə şansınızı sınayın. Sunny coin: hold the spin slot bonusları çox maraqlıdır.
Sunny Coin Hold The Spin pulsuz demo yeni başlayanlar üçün əladır. Sunny coin 2 hold the spin slot oynamadan əvvəl demo sınayın.
Oyunu kəşf et [url=https://sunny-coin.com.az/]sunnycoin az[/url].
Sunny coin 2 hold the spin slot müasir dizaynla təqdim olunur. Sunny coin 2 hold the spin slot demo rahat giriş imkanı yaradır. Sunny coin 2 hold the spin slot istifadəçilərin sevimlisidir.
Sunny coin 2 hold the spin slot real pul ilə uğurludur. Sunny Coin Hold The Spin real pul üçün əlverişlidir.
Bu gün sunny coin hold the spin oynadım və xoş təəssürat qaldı. Sunny coin hold the spin oyna və pulsuz fırlanmaları yoxla.
Sunny coin: hold the spin slot RTP yüksək olduğu üçün sevilir. Sunny Coin Hold The Spin slotu yüksək keyfiyyətli qrafikaya malikdir.
Oyunun yeni versiyasını sınayın [url=https://sunny-coin.com.az/]Sunny coin 2: hold the spin slot[/url].
Sunny coin hold the spin slot online demo çox istifadəlidir. Sunny Coin Hold The Spin oyunu sürətli və maraqlıdır. Sunny coin: hold the spin slot RTP səviyyəsi yüksəkdir.
Sunny Coin Hold The Spin pulsuz demo çox faydalıdır. Sunny coin: hold the spin slot təcrübəsi həyəcanlıdır.
Corgislot Casino https://alemmar.cm-nazare.pt/offlin-gokhal-beveiliging-pastoor-herken-jouw-corgislot-casino-een-veilig-offlin-casino/
Bu gün sunny coin hold the spin oynadım və xoş təəssürat qaldı. Sunny coin 2 hold the spin slot oynamaq çox asandır.
Sunny Coin Hold The Spin 2 yeni versiyası çox maraqlıdır. Sunny Coin Hold The Spin real pul üçün çox faydalıdır.
Daha maraqlı təcrübə üçün klik edin [url=https://sunny-coin.com.az/]sunny coin 2: hold the spin[/url].
Sunny coin 2: hold the spin slot pulsuz versiya rahatdır. Sunny Coin 2: Hold The Spin slotu çox realistik görünür. Sunny coin 2 hold the spin slot istifadəçilərin sevimlisidir.
Sunny coin hold the spin slot dizaynı çox rəngarəngdir. Sunny coin hold the spin slot oynamaq çox rahatdır.
I like to spend my free time by scanning various internet resources. Today I came across your website and I found it has some of the most practical and helpful information I’ve seen.
Проблемы с откачкой? насос для откачки грязной воды сдаем в аренду мотопомпы и вакуумные установки: осушение котлованов, подвалов, септиков. Производительность до 2000 л/мин, шланги O50–100. Быстрый выезд по городу и области, помощь в подборе. Суточные тарифы, скидки на долгий срок.
buy weed prague weed in prague
prague drugstore prague drugs
buy cocaine prague columbian cocain in prague
weed in prague coke in prague
Zivjeti u Crnoj Gori? https://www.placevi-zabljak-nekretnine.com Novi apartmani, gotove kuce, zemljisne parcele. Bez skrivenih provizija, trzisna procjena, pregovori sa vlasnikom. Pomoci cemo da otvorite racun, zakljucite kupoprodaju i aktivirate servis izdavanja. Pisite — poslacemo vam varijante.
Смотрите онлайн советские мультики смотреть онлайн лучшие детские мультфильмы, сказки и мульсериалы. Добрые истории, веселые приключения и любимые герои для малышей и школьников. Удобный поиск, качественное видео и круглосуточный доступ без ограничений.
Портал о строительстве https://gidfundament.ru и ремонте: обзоры материалов, сравнение цен, рейтинг подрядчиков, тендерная площадка, сметные калькуляторы, образцы договоров и акты. Актуальные ГОСТ/СП, инструкции, лайфхаки и готовые решения для дома и бизнеса.
Всё о ремонте https://remontkit.ru и строительстве: технологии, нормы, сметы, каталоги материалов и инструментов. Дизайн-идеи для квартиры и дома, цветовые схемы, 3D-планы, кейсы и ошибки. Подрядчики, прайсы, калькуляторы и советы экспертов для экономии бюджета.
Мир гаджетов https://indevices.ru новости, обзоры и тесты смартфонов, ноутбуков, наушников и умного дома. Сравнения, рейтинги автономности, фото/видео-примеры, цены и акции. Поможем выбрать устройство под задачи и бюджет. Подписка на новые релизы.
Женский портал https://art-matita.ru о жизни и балансе: модные идеи, уход за кожей и волосами, здоровье, йога и фитнес, отношения и семья. Рецепты, чек-листы, антистресс-практики, полезные сервисы и календарь событий.
Все автоновинки https://myrexton.ru премьеры, тест-драйвы, характеристики, цены и даты продаж. Электромобили, гибриды, кроссоверы и спорткары. Фото, видео, сравнения с конкурентами, конфигуратор и уведомления о старте приема заказов.
Bu gün sunny coin hold the spin oynadım və xoş təəssürat qaldı. Sunny coin hold the spin oyna və pulsuz fırlanmaları yoxla.
Sunny coin hold the spin slot online casino üçün ideal seçimdir. Sunny Coin Hold The Spin real pul üçün çox faydalıdır.
Rəsmi platforma [url=https://sunny-coin.com.az/]https://sunny-coin.com.az[/url].
Sunny Coin 2 hold the spin slot bonus imkanları çox genişdir. Sunny Coin 2 hold the spin slot real qələbə gətirə bilər. Sunny coin hold the spin slot online rahat oynanır.
Sunny Coin 2: Hold The Spin slot bonus funksiyaları əladır. Sunny Coin Hold The Spin oyunu hər büdcəyə uyğundur.
Новостной портал https://daily-inform.ru главные события дня, репортажи, аналитика, интервью и мнения экспертов. Лента 24/7, проверка фактов, региональные и мировые темы, экономика, технологии, спорт и культура.
Всё о стройке https://lesnayaskazka74.ru и ремонте: технологии, нормы, сметы и планирование. Каталог компаний, аренда техники, тендерная площадка, прайс-мониторинг. Калькуляторы, чек-листы, инструкции и видеоуроки для застройщиков, подрядчиков и частных мастеров.
Актуальные новости https://pr-planet.ru без лишнего шума: политика, экономика, общество, наука, культура и спорт. Оперативная лента 24/7, инфографика,подборки дня, мнения экспертов и расследования.
Строительный портал https://nastil69.ru новости, аналитика, обзоры материалов и техники, каталог поставщиков и подрядчиков, тендеры и прайсы. Сметные калькуляторы, ГОСТ/СП, шаблоны договоров, кейсы и лайфхаки.
Ремонт и стройка https://stroimsami.online без лишних затрат: гайды, сметы, план-графики, выбор подрядчика и инструмента. Честные обзоры, сравнения, лайфхаки и чек-листы. От отделки до инженерии — поможем спланировать, рассчитать и довести проект до результата.
ламінат тернопіль ціна https://remontuem.te.ua
Tremendous things here. I’m very satisfied to peer your post. Thank you so much and I am having a look forward to contact you. Will you please drop me a e-mail?
https://http-kra40.cc
значки заказные железные значки на заказ
изготовление значков из металла заказать значки с логотипом недорого
значки изготовить значок на лацкан пиджака на заказ
сайт для накрутки подписчиков в тг
Hello to every body, it’s my first go to see of this webpage; this web site consists of awesome and genuinely fine information for readers.
kra40 cc
Футболка и штаны и надписи на футболки на заказ в Челябинске. Макет худи для дизайна и пряжка для шарфа в Туле. Футболка со своим логотипом и футболка с принтом для мужчин во Владикавказе. Одежда из бамбука интернет и значок Z на одежду в Новосибирске. Ог буда футболка оптом и мужская футболка с принтом большого размера: цветная футболка с принтом оптом
накрутка подписчиков тг
Женские футболки с длинным рукавом и красивая печать на футболках в Костроме. Утепленную толстовку мужскую и женские брюки больших размеров в интернет недорого в Астрахани. Футболка лондон и белая футболка мужская без рисунка в Пензе. Ящики для упаковки подарков и этикетки для одежды в Тамбове. Парные футболки оптом для влюбленных надписи и футболка княZz: создать принт на футболки оптом
Надпись на футболку сколько стоит и мужские белые футболки с принтом в Брянске. Мужская рубашка большого размера и женская рубашка поло в Вологде. Надписи для футболки на английском и футболки со львом мужские в Якутске. Нонейм одежда и а4 мерч одежда в Костроме. Вышивка рисунков на футболке оптом и футболки мужские больших размеров https://futbolki-s-printom.ru/
накрутка подписчиков в тг группу
накрутить подписчиков в тг
the lucky cat maneki neko oyunda uğur simvoludur. Real pul üçün sınayın: [url=https://lucky-neko.com.az/]lucky neko casino[/url]. lucky neko gigablox slot daha həyəcanlıdır.
lucky neko slot demo yeni oyunçular üçün uyğundur. demo lucky neko pulsuz test üçündür. Burada oynayın: [url=https://lucky-neko.com.az/]http://lucky-neko.com.az[/url]. lucky neko demo slot risksiz sınaq verir. slot demo lucky neko keyfiyyətli animasiyalara malikdir. maneki neko lucky cat qədim ənənələrdən gəlir. demo lucky neko real pul oyununa hazırlayır. lucky neko casino real pul üçün seçimdir.
joszaki regisztracio joszaki.hu/
joszaki regisztracio joszaki
накрутка активных подписчиков в тг
joszaki regisztracio https://joszaki.hu/
Bu gün lucky neko demo oynadım və çox bəyəndim. Real pul üçün sınayın: [url=https://lucky-neko.com.az/]lucky neko casino[/url]. japanese lucky cat maneki neko mədəniyyəti əks etdirir.
maneki neko lucky cat uğur rəmzidir. maneki-neko lucky cat qədim simvoldur. Bütün məlumat üçün: [url=https://lucky-neko.com.az/]lucky-neko.com.az[/url]. maneki neko donkey lucky cat simvolu şənlik qatır. lucky neko slot demo çox məşhur seçimdir. lucky cat maneki neko uğur simvoludur. pg soft lucky neko funksiyaları çox zəngindir. lucky neko gigablox slot əyləncəni artırır.
Lucky Neko slot haqqında çoxlu faydalı məlumat tapdım. Real pul üçün sınayın: [url=https://lucky-neko.com.az/]lucky neko casino[/url]. lucky cat maneki neko uğur gətirən əfsanədir.
lucky neko cat slotunda qrafika yüksək keyfiyyətlidir. neko lucky cat slotda uğuru simvollaşdırır. Demo üçün birbaşa link: [url=https://lucky-neko.com.az/]https://lucky-neko.com.az[/url]. lucky neko slot demo bonuslarla maraqlıdır. lucky neko pg slot çox asan oynanır. slot lucky neko demo oynamaq çox əyləncəlidir. neko lucky oyunun dizaynı çox rəngarəngdir. pg soft lucky neko çoxlu bonuslar verir.
Lucky Neko slot haqqında çoxlu faydalı məlumat tapdım. Pulsuz test üçün klikləyin: [url=https://lucky-neko.com.az/]lucky neko demo[/url]. como jogar lucky neko çox sadədir.
lucky cat lucky cat maneki neko kolleksiyası maraqlıdır. neko lucky cat slotda uğuru simvollaşdırır. Bütün məlumat üçün: [url=https://lucky-neko.com.az/]lucky-neko.com.az[/url]. lucky neko slot qrafikası çox müasir görünür. pg lucky neko demo versiyası yeni istifadəçilər üçündür. maneki neko lucky cat tiki mug kolleksiyası məşhurdur. neko lucky oyunun dizaynı çox rəngarəngdir. lucky neko demo slot vaxt keçirmək üçün əladır.
накрутка подписчиков в тг платно
смм накрутка подписчиков тг
the lucky cat maneki neko oyunda uğur simvoludur. Tamamilə pulsuz sınayın: [url=https://lucky-neko.com.az/]demo lucky neko gratis[/url]. como jogar lucky neko çox sadədir.
lucky cat lucky cat maneki neko kolleksiyası maraqlıdır. pg soft demo lucky neko sayəsində risksiz sınaq mümkündür. Rəsmi giriş: [url=https://lucky-neko.com.az/]www.lucky-neko.com.az sayt[/url]. maneki neko donkey lucky cat simvolu şənlik qatır. lucky neko pg slot çox asan oynanır. pg soft lucky neko çox maraqlı oyundur. lucky cat maneki-neko uğur gətirən simvoldur. slot demo lucky neko çoxlu imkanlar açır.
Индивидуалка приехала быстро, выглядела шикарно, словно модель из журнала. Разговор легкий, улыбка искренняя, руки нежные. Было невероятно приятно и комфортно. Рекомендую, шлюхи вызвать Новосиб – https://sibirki3.vip/. Настоящий отдых для души и тела.
накрутить подписчиков в телеграм канал живых
Estou alucinado com MarjoSports Casino, pulsa com uma forca de cassino digna de um craque. Tem uma enxurrada de jogos de cassino irados. incluindo jogos de mesa com um toque de estrategia. O atendimento esta sempre ativo 24/7. garantindo suporte direto e sem impedimentos. As transacoes sao faceis como um apito. as vezes as ofertas podiam ser mais generosas. Em sintese, MarjoSports Casino e o point perfeito pros fas de cassino para quem curte apostar com estilo esportivo! Alem disso o layout e vibrante como um apito. criando uma experiencia de cassino de torcida.
marjosports ganhe 10 reais|
Galera, nao podia deixar de comentar no 4PlayBet Casino porque me impressionou bastante. A variedade de jogos e surreal: roletas animadas, todos funcionando perfeito. O suporte foi eficiente, responderam em minutos pelo chat, algo que passa seguranca. Fiz saque em Bitcoin e o dinheiro entrou mais ligeiro do que imaginei, ponto fortissimo. Se tivesse que criticar, diria que podia ter mais promocoes semanais, mas isso nao estraga a experiencia. Enfim, o 4PlayBet Casino e completo. Ja virou parte da minha rotina.
4play ПѓП…ОіОєПЃОїП„О·ОјО±|
Me encantei pelo ritmo de BR4Bet Casino, e um cassino online que reluz como um farol na nevoa. Os jogos formam um clarao de entretenimento. incluindo jogos de mesa com um toque de brilho. Os agentes sao rapidos como um raio de farol. oferecendo respostas claras como um farol. As transacoes sao simples como uma luz. em alguns momentos mais recompensas fariam o coracao brilhar. Na real, BR4Bet Casino oferece uma experiencia que e puro brilho para os faroleiros do cassino! Por sinal o visual e uma explosao de claroes. adicionando um toque de luz ao cassino.
[br4bet]|
купить живых подписчиков в тг
сколько стоит подписчик в тг канал
накрутка подписчиков в группу телеграм
подписчики в тг канал
как накрутить подписчиков в телеграм канале
накрутка живых подписчиков в тг
jogar lucky neko demo öyrənmək üçün rahatdır. Populyar oyunu buradan kəşf edin: [url=https://lucky-neko.com.az/]pg lucky neko[/url]. lucky neko gigablox slot daha həyəcanlıdır.
maneki neko lucky cat ceramic tiki mug kolleksiya üçün əladır. lucky cat maneki neko tattoo maraqlı görünür. Əlavə link: [url=https://lucky-neko.com.az/]lucky neko com az[/url]. lucky neko demo slot risksiz sınaq verir. pg lucky neko demo versiyası yeni istifadəçilər üçündür. pg soft lucky neko çox maraqlı oyundur. pg soft lucky neko funksiyaları çox zəngindir. pg soft demo lucky neko başlanğıc üçün uyğundur.
накрутка настоящих подписчиков в тг
накрутка подписчиков в тг канал живые
накрутить подписчиков в тг
онлайн накрутка подписчиков тг
Me encantei pelo fulgor de Verabet Casino, tem uma energia de jogo tao intensa quanto um ritual de fogo. A selecao de titulos e uma pira de emocoes. com caca-niqueis que reluzem como brasas. O servico e confiavel como uma fogueira. oferecendo respostas claras como uma chama. As transacoes sao faceis como um fulgor. porem queria mais promocoes que queimam como fogueiras. Resumindo, Verabet Casino vale explorar esse cassino ja para os fas de adrenalina ardente! Vale dizer o layout e vibrante como uma tocha. amplificando o jogo com vibracao ardente.
bet vera e confiГЎvel|
купить айфон
накрутка подписчиков в тг бесплатно онлайн
motocitee – I’d return here to explore more, seems like content worth following.
Certainly. And I have faced it. Let’s discuss this question. Here or in PM.
Formula 1 izləmək üçün hansı kanalları seçmək barədə tövsiyələr. Formula 1 Bakıda 2025 tarixlərini indi öyrənin.
Yarış təqvimi və nəticələr üçün baxın ➡ [url=https://formula-1.com.az/]formula-1 com az[/url].
Formula 1 haqqinda melumat axtaranlar üçün faydalı mənbə. Formula 1 film və sənədli layihələrinə baxmaq mümkündür.
Formula 1 Baku track barədə dəqiq xəritə məlumatı. Formula 1 volunteer olmaq istəyənlər üçün təcrübə. Formula 1 movie izləyicilər üçün əyləncəli seçimdir. Formula 1 drivers ilə bağlı ən son xəbərlər.
Sou viciado no labirinto de IJogo Casino, oferece uma aventura que se entrelaca como uma rede tropical. As escolhas sao vibrantes como um cipo. com jogos adaptados para criptomoedas. Os agentes sao rapidos como uma cobra. respondendo rapido como um cipo se enrolando. Os saques deslizam como cipos. as vezes mais bonus regulares seriam selvagens. Em resumo, IJogo Casino e um cassino online que e um labirinto de diversao para os viciados em emocoes de cassino! De bonus o layout e vibrante como uma raiz. fazendo o cassino se entrelacar como uma teia.
ijogo do flamengo|
Me ecoei no ritmo de Stake Casino, parece uma camara de ecos cheia de adrenalina. O leque do cassino e um reverb de delicias. com slots tematicos de aventuras sonoras. O time do cassino e digno de um maestro. com ajuda que ressoa como um sino. O processo e claro e sem pausas. porem mais recompensas fariam o coracao ecoar. Na real, Stake Casino e um cassino online que e uma camara de diversao para os cacadores de vitorias ressonantes! E mais o visual e uma explosao de sinos. adicionando um toque de eco ao cassino.
my stake|
Galera, nao podia deixar de comentar sobre o Bingoemcasa porque me ganhou de verdade. O site tem um jeito leve que lembra um barzinho cheio de risadas. As salas de bingo sao com muita interacao, e ainda testei alguns caca-niqueis modernos, todos foram bem estaveis. O atendimento no chat foi educado e prestativo, o que ja me deixou bem a vontade. As retiradas foram sem enrolacao, inclusive testei cripto e nao tive problema nenhum. Se pudesse apontar algo, diria que faltam mais promocoes, mas nada que estrague a experiencia. Pra concluir, o Bingoemcasa me conquistou. Ja me sinto parte da comunidade
bingoemcasa site|
Play the lottery game https://lemon-cazino-pl.com
Lottery game https://betvisabengal.com
Cleaning is needed cleaning toronto eco-friendly supplies, vetted cleaners, flat pricing, online booking, same-day options. Bonded & insured crews, flexible scheduling. Book in 60 seconds—no hidden fees.
Портал Чернівців https://58000.com.ua оперативні новини, анонси культурних, громадських та спортивних подій, репортажі з міста, інтерв’ю з чернівчанами та цікаві історії. Все про життя Чернівців — щодня, просто й доступно
Formula 1 canlı izləmə imkanı ilə yarışları qaçırmayın. Ən yaxşı Formula 1 filmləri və sənədli layihələri.
Ən yaxşı nəticələr və statistikalar üçün baxın ➡ [url=https://formula-1.com.az/]formula 1 standings[/url].
Formula 1 Türkiye mərhələsi haqqında ətraflı yazılar. Formula 1 puan cədvəli ilə favoritlərinizi izləyin.
Formula 1 2025 calendar üzrə bütün yarış tarixləri. Formula 1 puan durumu ilə komandaları izləyin. Formula 1 canlı izləmə təcrübənizi paylaşın. Formula 1 azerbaycanda keçirilən yarışlar barədə maraqlı faktlar.
накрутка живых подписчиков телеграм канал
Formula 1 2025 təqvimini öyrənmək istəyənlər üçün faydalı məlumat. Bakı Formula 1 xəritəsi üzrə hər bir döngəni tanıyın.
Formula 1 barədə hər şey burada ➡ [url=https://formula-1.com.az/]www.formula-1.com.az[/url].
Formula 1 haqqinda melumat axtaranlar üçün faydalı mənbə. Formula 1 Bakı 2024 biletləri onlayn əldə oluna bilər.
Formula 1 haqqında maraqlı tarixi faktlar. Formula 1 2025 Baku mərhələsi barədə ən son xəbərlər. Formula 1 movie izləyicilər üçün əyləncəli seçimdir. Formula 1 2025 Baku üçün təqvim yenilənib.
бот для накрутки подписчиков в телеграм
Formula 1 yarışları haqqında ən son xəbərləri burada izləyin. Bakı Formula 1 xəritəsi üzrə hər bir döngəni tanıyın.
Ən son xəbərləri izləmək üçün baxın ➡ [url=https://formula-1.com.az/]formula 1[/url].
Formula 1 canli yayım üçün yeni platformalar. Formula 1 təqvimində dəyişikliklər barədə xəbərlər.
Formula 1 drivers siyahısı daim dəqiqləşdirilir. Formula 1 Azərbaycan mərhələsinin atmosferi barədə şərhlər. Formula 1 volunteer 2025 qeydiyyatı başladı. Formula 1 2025 Baku üçün təqvim yenilənib.
Formula 1 könüllü proqramı haqqında maraqlı məlumatlar burada. Formula 1 üçün ən yaxşı bilet seçimlərini tapmaq mümkündür.
Yarışlarla bağlı ətraflı məlumat üçün ➡ [url=https://formula-1.com.az/]http://formula-1.com.az[/url].
Formula 1 canlı izləmə ilə bağlı bütün təcrübələr. Formula 1 Baku 2025 üçün ən maraqlı məlumatlar.
Formula 1 2025 calendar üzrə bütün yarış tarixləri. Formula 1 volunteer olmaq istəyənlər üçün təcrübə. Formula 1 izləyiciləri üçün ən son xəbərlər. Formula 1 baku 2024 tickets artıq satışdadır.
смм накрутка подписчиков тг
активные подписчики в тг канал
Reliable Briansclub.ga credit supports better financial strategies.
My brother suggested I might like this web site. He was entirely right. This post actually made my day. You can not imagine simply how much time I had spent for this info! Thanks!
смм накрутка подписчиков телеграм
Me encantei pelo ritmo de DonaldBet Casino, parece um espetaculo de adrenalina colorida. As opcoes sao ricas e reluzem como malabares. oferecendo sessoes ao vivo que brilham como holofotes. Os agentes voam como trapezistas. com solucoes precisas e instantaneas. Os saques sao velozes como um trapezio. mas queria promocoes que explodem como fogos. Para encurtar, DonaldBet Casino e um picadeiro de emocoes para os viciados em emocoes de cassino! Adicionalmente a navegacao e facil como um malabarismo. adicionando um toque de magia de circo ao cassino.
afiliado donaldbet|
Estou alucinado com JonBet Casino, parece uma camara de ecos cheia de adrenalina. A colecao e uma onda sonora de entretenimento. com caca-niqueis modernos que ecoam como sinos. O servico e confiavel como uma camara. com ajuda que ressoa como um sino. Os pagamentos sao seguros e fluidos. porem mais bonus seriam um diferencial ressonante. Resumindo, JonBet Casino vale explorar esse cassino ja para os cacadores de vitorias ressonantes! Como extra o site e uma obra-prima de estilo sonoro. criando uma experiencia de cassino harmonica.
jonbet esporte|
Je trouve absolument boomerang Boomerang Casino, on dirait un cercle de frissons qui boucle. L’assortiment de jeux du casino est une courbe de delices. incluant des tables qui ricochet comme un projectile. est un virtuose du cercle. resonnant comme une boucle parfaite. Les paiements du casino sont securises et fluides. par moments des tours gratuits pour une melodie ricochet. Dans l’ensemble, Boomerang Casino enchante avec une rhapsodie de jeux pour les explorateurs de melodies en ligne! Ajoutons resonne avec une melodie graphique arque. ajoute une touche de rythme arque au casino.
boomerang casino mobiili|
накрутка подписчиков тг
Formula 1 izləmək üçün hansı kanalları seçmək barədə tövsiyələr. Formula 1 üçün ən yaxşı bilet seçimlərini tapmaq mümkündür.
Bakı mərhələsi barədə bütün məlumatlar ➡ [url=https://formula-1.com.az/]formula 1 baku[/url].
Formula 1 Türkiye mərhələsi haqqında ətraflı yazılar. Formula 1 Bakı 2024 biletləri onlayn əldə oluna bilər.
Formula 1 schedule izləyicilər üçün rahat seçimdir. Formula 1 baku haqqinda melumat axtaranlara məsləhətlər. Formula 1 2024 takvimi ilə bağlı maraqlı dəyişikliklər. Formula 1 puan durumu ilə favorit komandanızı izləyin.
накрутка подписчиков тг канале бесплатно без отписок
usa casino providers, tiger gaming poker uk and online casinos that accept apple
pay usa, or fastest withdrawal online casino canada no deposit bonus
my page :: best places to gamble in vegas
На складе крупного дистрибьютора мы внедрили оборудование Labelaire, а также терминалы сбора данных. Это помогло оптимизировать инвентаризацию и ускорить процессы. Дополнительно используем сканеры штрих-кодов для приёмки и отгрузки. Теперь все данные максимально точные и всегда доступны: https://labelaire.ru/
как безопасно накрутить подписчиков в телеграм
Установка и монтаж видеонаблюдения https://vcctv.ru
подписчики в тг канал реклама
інформаційний портал https://01001.com.ua Києва: актуальні новини, політика, культура, життя міста. Анонси подій, репортажі з вулиць, інтерв’ю з киянами, аналітика та гід по місту. Все, що треба знати про Київ — щодня, просто й цікаво.
інформаційний портал https://65000.com.ua Одеси та регіону: свіжі новини, культурні, громадські та спортивні події, репортажі з вулиць, інтерв’ю з одеситами. Всі важливі зміни та цікаві історії про життя міста — у зручному форматі щодня
LuckyMax NL https://mariscosmardearousa.es/online-luckymax-casino-holland-uitgelezene-legale-casinos-voor-2024/
Je suis hante par Casombie, il offre une aventure aussi sombre que palpitante. La gamme est un veritable apocalypse de fun, avec des slots au design effrayant. Le service client est d’une efficacite surnaturelle, avec une aide aussi fluide qu’un spectre. Les paiements sont securises comme une tombe scellee, parfois plus de promos macabres seraient un plus. Au final, Casombie est un must pour les amateurs de sensations fortes pour ceux qui aiment l’adrenaline sombre ! A noter le site est visuellement un chef-d’?uvre macabre, donne envie de revenir hanter le site.
casombie vГ©lemГ©nyek|
Je suis totalement hypnotise par Freespin Casino, ca scintille comme une aurore boreale. Les options forment un tourbillon de surprises, incluant des jeux de table d’une energie debordante. Le service client est d’une efficacite siderante, avec une aide aussi fluide qu’une brise etoilee. Les paiements sont securises comme une voute celeste, de temps a autre plus de promos vibrantes seraient un regal. En somme, Freespin Casino promet une aventure etincelante pour les fans de casinos en ligne ! Cerise sur le gateau le design est vibrant comme une galaxie, donne envie de replonger dans l’univers du jeu.
casino free spin no deposit 2020|
Je trouve absolument spectral Nomini Casino, est une symphonie de divertissement qui effleure. La selection du casino est une danse de plaisirs. offrant des lives qui pulsent comme un theatre. Le personnel du casino offre un accompagnement digne d’un directeur de scene. offrant des solutions claires et instantanees. arrivent comme un concerto voile. mais des bonus de casino plus frequents seraient spectrale. En conclusion, Nomini Casino est un joyau pour les fans de casino pour les danseurs spectraux du casino! De plus est une cadence visuelle envoutante. ce qui rend chaque session de casino encore plus etheree.
J’eprouve une gourmandise infinie pour MrPacho Casino, ca infuse un univers de saveurs ludiques. Le menu est un cellier de variete exuberante, integrant des live roulettes pour des tourbillons de suspense. Le service officie en continu 24/7, distillant des remedes clairs et prompts. Les flux monetaires sont blindes par des epices crypto, par eclats les menus d’offres pourraient s’etoffer en generosite. En apotheose culinaire, MrPacho Casino tisse une tapisserie de divertissement gustatif pour les gardiens des buffets numeriques ! A souligner la trame irradie comme un plat ancestral, pousse a prolonger le banquet infini.
mrpacho seriös|
купить накрутку подписчиков тг
накрутка подписчиков в тг бесплатно и быстро
new 2021 usa players online casino, claiming gambling winnings
in united kingdom and best united kingdom casino, or online poker
with united statesn funds
My web-site … Goplayslots.net
Я читаю theHold уже несколько месяцев и вижу огромную пользу. Прогнозы часто совпадают с реальной динамикой рынка, новости актуальные, а калькулятор доходности помогает планировать инвестиции. Материалы написаны простым языком, это делает журнал удобным для изучения – https://thehold.ru/
J’ai un veritable coup de c?ur pour Cheri Casino, ca scintille comme une nuit etoilee. Le catalogue est une mosaique de plaisirs, incluant des jeux de table pleins de charme. Le service client est d’une douceur angelique, avec une aide aussi chaleureuse qu’un rayon de soleil. Les paiements sont securises comme un coffre-fort, parfois les offres pourraient etre plus flamboyantes. Dans l’ensemble, Cheri Casino merite d’etre explore sans tarder pour les amoureux des cryptos ! Par ailleurs la navigation est intuitive comme une danse, amplifie l’immersion dans un monde de reves.
avis sur cheri casino|
Je suis aromatise par PepperMill Casino, il concocte une alchimie de gains epoustouflants. Le bouquet est un potager de diversite exuberante, incluant des jackpots progressifs pour des pics d’essence. L’assistance distille des elixirs affutes, avec une expertise qui presage les appetits. Le processus est moulu pour une onctuosite exemplaire, par bouffees des elans promotionnels plus assidus animeraient le jardin. Dans la totalite du bouquet, PepperMill Casino tisse une tapisserie de divertissement olfactif pour les gardiens des jardins numeriques ! En piment sur le gateau la trame irradie comme un elixir ancestral, pousse a prolonger le festin infini.
peppermill reno login|
Je suis fleche par WildRobin Casino, il decoche une salve de gains inattendus. Il grouille d’une horde de quetes interactives, integrant des lives comme Blackjack pour des duels d’arcs. L’assistance decoche des reponses precises, bandant des cordes multiples pour une frappe immediate. Les transferts sprintent stables et acceleres, a l’occasion les salves d’offres pourraient s’epaissir en densite. A la fin de cette quete, WildRobin Casino devoile un sentier de triomphes inattendus pour ceux qui visent leur destin en ligne ! Par surcroit la structure vibre comme un arc ancestral, infuse une essence de mystere sylvestre.
wild robin 2|
Top topics and trends: https://joogastuudio.ee
Smart crypto trading https://terionbot.com with auto-following and DCA: bots, rebalancing, stop-losses, and take-profits. Portfolio tailored to your risk profile, backtesting, exchange APIs, and cold storage. Transparent analytics and notifications.
Everything you need is here: https://lesma-shop.de
Soccer tətbiqi futbol həvəskarları üçün idealdır.
Opera soccer tətbiqi ilə xəbərlərə tez çatın. Futbol xəbərləri və nəticələr üçün [url=https://soccer.com.az/]soccer 365[/url] rahat mənbədir.
Super liquid soccer fərqli futbol təcrübəsi bəxş edir. Soccer champs hile versiyaları əlavə üstünlüklər yaradır. Soccer legends klassik futbol anlarını yaşadır.
Soccer skills champions league ilə yeni bacarıqlar öyrənin. Soccer field dizaynı oyunda çox real görsənir. Spbo live score soccer ilə hər dəqiqə nəticələri görün. World soccer champs apk 8.3.2 para hilesi ilə resurs artırın.
LuckyMax NL https://www.arasgrupinsaat.com/offlin-gokhuis-nederlan-beste-legale-casinos-luckymax-casino-voor-2024/
https://clickolov.ru/
Mobil cihazlarda soccer oyunu yüksək keyfiyyətlə işləyir.
Mini soccer star mod apk futbol oynamaq üçün əla seçimdir. Canlı matç izləmək istəyənlər üçün ən yaxşı seçim [url=https://soccer.com.az/]live soccer[/url] bölməsidir.
Soccer live tətbiqi ilə canlı izləmə daha rahatdır. Rocket soccer derby fərqli futbol janrını təqdim edir. Soccer predictions futbol nəticələrini təxmin etməyə kömək edir.
Pro direct soccer məhsulları keyfiyyətli seçimdir. Soccer manager 2025 hile ilə əlavə imkanlar mövcuddur. World soccer champs apk son sürüm daha yaxşı işləyir. Soccer star mod apk ilə daha çox imkan əldə edin.
The sketch is tasteful, your authored material stylish.
Mobil soccer yükləyərək futbol əyləncəsini hər yerdə yaşayın.
Opera soccer tətbiqi ilə xəbərlərə tez çatın. Beynəlxalq turnirlər barədə ətraflı oxumaq üçün [url=https://soccer.com.az/]globe soccer[/url] bölməsi faydalıdır.
World soccer champs mod apk ilə əlavə imkanlar əldə edin. Pro soccer online multiplayer həvəskarları üçün uyğundur. Soccer legends klassik futbol anlarını yaşadır.
Soccer super star mod apk ilə ulduz olun. Soccer champs apk yükləmək çox asandır. World soccer champs apk son sürüm daha yaxşı işləyir. World soccer futbol sevərlərin əvəzolunmaz seçimidir.
Soccer oyununda komandalar seçmək çox asandır.
Flashscore mobile livescore today soccer ilə ani nəticələr alın. Mobil futbol oyunları üçün əsas ünvan [url=https://soccer.com.az/]soccer com az[/url] portalıdır.
Soccer champs mod apk ilə oyun daha maraqlı olur. Soccer star oyunçular üçün motivasiyaedici seçimdir. Soccer manager 2025 mod apk ilə komandanızı idarə edin.
Pro direct soccer məhsulları keyfiyyətli seçimdir. Fifa soccer mod apk team2earn futbolu daha real edir. Soccer live score ilə canlı nəticələri izləyin. Soccer legends futbolun tarixi anlarını oyuna gətirir.
I’ve read several good stuff here. Definitely worth bookmarking for revisiting. I surprise how much effort you put to make such a magnificent informative site.
Je suis irremediablement appate par MrPacho Casino, on discerne un jardin de defis appetissants. La vitrine de jeux est un buffet opulent de plus de 5 000 delices, incluant des crash games comme JetX pour des pics de saveur. Les hotes interviennent avec une delicatesse remarquable, avec une maestria qui anticipe les appetits. Les flux monetaires sont blindes par des epices crypto, bien qu’ des garnitures de recompense additionnelles epiceraient les alliances. En apotheose culinaire, MrPacho Casino devoile un itineraire de triomphes succulents pour les chasseurs de casinos virtuels ! Par surcroit la trame irradie comme un plat ancestral, instille une essence de mystere culinaire.
mrpacho casino en ligne|
J’eprouve un vertige total pour Shuffle Casino, on anticipe un paquet de ruses astucieuses. Il foisonne d’une ribambelle de tirages interactifs, offrant des loteries SHFL avec des pots jusqu’a 2 millions des maitres comme Pragmatic Play. Le service pioche en continu 24/7, tirant des solutions claires et rapides. Le protocole est melange pour une fluidite exemplaire, bien que des tirages gratuits supplementaires brasseraient les mains. En apotheose hasardeuse, Shuffle Casino se pose comme un atout pour les parieurs pour les tricheurs des paris crypto ! A piocher l’interface est un tapis navigable avec ruse, amplifie l’engagement dans le casino du hasard.
casino wash shuffle|
I saw a similar post on another website but the points were not as well articulated.
LuckyMax NL https://scuolaitalianabucarest.com/nieuwste-online-bank-holland-luckymax-2024-nieuwe-casinos/
Je suis enrobe par Sugar Casino, on savoure un comptoir de tactiques allechantes. Les choix forment un caramel de saveurs innovantes, proposant des roulettes pour des tours de sucre. Les artisans repondent avec une douceur remarquable, avec une expertise qui anticipe les envies. Les flux financiers sont enrobes par des coques crypto, occasionnellement les offres pourraient s’epaissir en generosite. Dans l’ensemble de la confiserie, Sugar Casino se dresse comme un pilier pour les gourmands pour les artisans de victoires sucrees ! En plus la circulation est instinctive comme un coulis, incite a prolonger la degustation infinie.
sugar casino erfahrung|
Android üçün ən yaxşı soccer tətbiqini sınayın.
Flashscore mobile livescore today soccer ilə ani nəticələr alın. Mobil futbol oyunları üçün əsas ünvan [url=https://soccer.com.az/]soccer com az[/url] portalıdır.
Super liquid soccer fərqli futbol təcrübəsi bəxş edir. Livescore soccer results futbol azarkeşləri üçün vacibdir. Real soccer 2012 nostalji sevənlər üçün uyğundur.
Soccer skills champions league ilə yeni bacarıqlar öyrənin. World soccer champs maç atlama hilesi ilə oyun sürətlənir. Soccer super star futbolçular üçün maraqlıdır. World soccer champs apk 8.3.2 para hilesi ilə resurs artırın.
Обратились за SEO продвижением, так как сайт практически не приносил заявок. Специалисты сделали полный аудит, исправили ошибки, доработали структуру и добавили оптимизированный контент. Уже через пару месяцев пошёл стабильный рост трафика и звонков. Теперь мы уверенно обходим конкурентов и видим реальную отдачу от вложений – https://mihaylov.digital/
Сломалась машина? помощь на дорогах спб мы создали профессиональную службу автопомощи, которая неустанно следит за безопасностью автомобилистов в Санкт-Петербурге и Ленинградской области. Наши специалисты всегда на страже вашего спокойствия. В случае любой нештатной ситуации — от банальной разрядки аккумулятора до серьёзных технических неисправностей — мы незамедлительно выезжаем на место.
LuckyMax https://server-test.momilash.com/online-casino-nederlan-u-lieve-va-lucky-max-casino-nu//
iOS üçün soccer yükləyib pulsuz oynayın.
Soccer 365 ilə futbol xəbərlərindən xəbərdar olun. Ən son yenilikləri öyrənmək üçün [url=https://soccer.com.az/]soccer.com.az[/url] linkinə baxa bilərsiniz.
Globe soccer turnirləri ilə dünya futboluna yaxın olun. Soccer star oyunçular üçün motivasiyaedici seçimdir. Soccer bros multiplayer futbol üçün əla seçimdir.
Dream league soccer 2025 para və elmas hilesi ilə daha çox fürsət əldə edin. Soccer champs apk yükləmək çox asandır. Pro evolution soccer 2019 hələ də çox oynanır. Mini soccer star apk sadə və əyləncəlidir.
Je suis enivre par MrPacho Casino, ca transforme le jeu en une degustation exquise. La vitrine de jeux est un buffet opulent de plus de 5 000 delices, integrant des live roulettes pour des tourbillons de suspense. Les hotes interviennent avec une delicatesse remarquable, avec une maestria qui anticipe les appetits. Les retraits glissent avec une souplesse remarquable, nonobstant les menus d’offres pourraient s’etoffer en generosite. Dans l’ensemble du menu, MrPacho Casino tisse une tapisserie de divertissement gustatif pour les architectes de victoires savoureuses ! A souligner le visuel est une mosaique dynamique et appetissante, instille une essence de mystere culinaire.
mrpacho online|
Мы доверили строительство дома под ключ компании СтройСинтез и не пожалели. Проект кирпичного дома с мансардой и гаражом был реализован без задержек. Дом выглядит современно, планировка продуманная. Очень довольны результатом, https://stroysyntez.com/
J’eprouve une majeste infinie pour SlotsPalace Casino, ca erige un empire de defis somptueux. Le royaume est un hall de diversite princiere, integrant des lives comme Infinite Blackjack pour des audiences de suspense. L’assistance proclame des edits nets, mobilisant des allegeances multiples pour une audience immediate. Les retraits s’executent avec une grace remarquable, malgre cela davantage de decrets bonus quotidiens enrichiraient le domaine. A la fin de cette ordonnance, SlotsPalace Casino devoile un arbre de triomphes opulents pour les architectes de victoires imperiales ! En sus l’interface est un couloir navigable avec splendeur, incite a prolonger la dynastie infinie.
informations slots palace|
Je suis pimente par PepperMill Casino, ca transfigure le jeu en une infusion eternelle. Les varietes tracent un panorama de saveurs novatrices, proposant des blackjacks revisites pour des bouffees d’excitation. Le service infuse en continu 24/7, accessible par infusion ou missive instantanee. Les courants financiers sont fortifies par des racines crypto, par intermittence les bouquets d’offres pourraient s’epanouir en generosite. A la fin de cette degustation, PepperMill Casino emerge comme un pilier pour les epicuriens pour les maitres de victoires odorantes ! En piment sur le gateau le portail est une serre visuelle imprenable, instille une quintessence de mystere epice.
peppermill casino belgique|
https://www.wildberries.ru/catalog/249860602/detail.aspx
Je suis propulse par Super Casino, ca catapulte le jeu vers un sommet heroique. Le stock est un manuel de divertissements surpuissants, avec des slots aux pouvoirs thematiques qui amplifient les combos. L’assistance deploie des tactiques affutees, declenchant des contre-mesures claires et rapides. Les trophees atterrissent via Bitcoin ou Ethereum, bien que davantage de boosts bonus quotidiens survoltent le bastion. Dans la globalite du QG, Super Casino devoile un vecteur de succes surhumains pour les sentinelles des bases numeriques ! A scanner la circulation est instinctive comme un jetpack, infuse une essence de mystere surhumain.
super cat casino free spins|
Мир гаджетов без воды https://indevices.ru честные обзоры, реальные замеры, фото/видео-примеры. Смартфоны, планшеты, аудио, гейминг, аксессуары. Сравнения моделей, советы по апгрейду, трекер цен и уведомления о скидках. Помогаем выбрать устройство под задачи.
Ремонт и стройка https://remontkit.ru без лишних затрат: инструкции, таблицы расхода, сравнение цен, контроль скрытых работ. База подрядчиков, отзывы, чек-листы, калькуляторы. Тренды дизайна, 3D-планировки, лайфхаки по хранению и зонированию. Практика и цифры.
Ваш портал о стройке https://gidfundament.ru и ремонте: материалы, инструменты, сметы и бюджеты. Готовые решения для кухни, ванной, спальни и террасы. Нормы, чертежи, контроль качества, приёмка работ. Подбор подрядчика, прайсы, акции и полезные образцы документов.
Все про ремонт https://lesnayaskazka74.ru и строительство: от идеи до сдачи. Пошаговые гайды, электрика и инженерия, отделка, фасады и кровля. Подбор подрядчиков, сметы, шаблоны актов и договоров. Дизайн-инспирации, палитры, мебель и свет.
Все про ремонт https://lesnayaskazka74.ru и строительство: от идеи до сдачи. Пошаговые гайды, электрика и инженерия, отделка, фасады и кровля. Подбор подрядчиков, сметы, шаблоны актов и договоров. Дизайн-инспирации, палитры, мебель и свет.
Ремонт и строительство https://nastil69.ru от А до Я: планирование, закупка, логистика, контроль и приёмка. Калькуляторы смет, типовые договора, инструкции по инженерным сетям. Каталог подрядчиков, отзывы, фото-примеры и советы по снижению бюджета проекта.
Нужен аккумулятор? аккумулятор на обмен с доставкой в наличии: топ-бренды, все размеры, правый/левый токовывод. Бесплатная проверка генератора при установке, trade-in старого АКБ. Гарантия до 3 лет, честные цены, быстрый самовывоз и курьер. Поможем выбрать за 3 минуты.
Хочешь сдать акб? прием акб честная цена за кг, моментальная выплата, официальная утилизация. Самовывоз от 1 шт. или приём на пункте, акт/квитанция. Безопасно и законно. Узнайте текущий тариф и ближайший адрес.
Мы выбрали кухню среднего сегмента. Она сочетает стиль, удобство и качество материалов https://kuhni-v-dom.ru/
Simply want to say your article is as amazing. The clarity in your post is just great and i can assume you’re an expert on this subject. Fine with your permission allow me to grab your feed to keep up to date with forthcoming post. Thanks a million and please keep up the gratifying work.
slot bonausaa hd, poker usa artrix and windsor casino in united
states, or online gambling revenue us (Jeannette) live roulette casino usa
кайт Кайт школа – это учебное заведение, специализирующееся на обучении кайтсёрфингу и другим видам спорта с кайтом. В кайт школах работают сертифицированные инструкторы, которые обучают основам управления кайтом, правилам безопасности и продвинутым техникам катания. Кайт школы предоставляют необходимое оборудование, включая кайты, доски, гидрокостюмы и шлемы. Обучение в кайт школе позволяет безопасно и эффективно освоить кайтсёрфинг и получить удовольствие от этого экстремального вида спорта.
АрендаАвто-мск https://mosavtomoto.ru прокат авто без водителя в Москве. Новый автопарк, выгодные тарифы, нулевая франшиза, страховка ОСАГО и КАСКО. Бизнес, премиум и эконом-класс. Быстрое бронирование и аренда в день обращения. Звоните: 8 495 2900095. Свобода движения в Москве!
Ищешь аккумулятор? аккумулятор для автомобиля AKB SHOP занимает лидирующие позиции среди интернет-магазинов автомобильных аккумуляторов в Санкт-Петербурге. Наш ассортимент охватывает все категории транспортных средств. Независимо от того, ищете ли вы надёжный аккумулятор для легкового автомобиля, мощного грузовика, комфортного катера, компактного скутера, современного погрузчика или специализированного штабелёра
Нужен надежный акб? купить аккумулятор для авто с доставкой AKB STORE — ведущий интернет-магазин автомобильных аккумуляторов в Санкт-Петербурге! Мы специализируемся на продаже качественных аккумуляторных батарей для самой разнообразной техники. В нашем каталоге вы найдёте идеальные решения для любого транспортного средства: будь то легковой или грузовой автомобиль, катер или лодка, скутер или мопед, погрузчик или штабелер.
Центр лицензирования Журавлев Консалтинг Групп помог оформить лицензию на медицинскую деятельность «под ключ», сотрудники проверили документы, проконсультировали по требованиям и сопровождали подачу до финального разрешения: https://licenz.pro/
v1av8 – Clean design elements, yet missing messaging that anchors the site.
Ich bin suchtig nach King Billy Casino, es ist ein Online-Casino, das wie ein Konig regiert. Die Auswahl im Casino ist ein wahres Kronungsjuwel, mit modernen Casino-Slots, die verzaubern. Der Casino-Support ist rund um die Uhr verfugbar, liefert klare und schnelle Losungen. Der Casino-Prozess ist klar und ohne Intrigen, ab und zu mehr regelma?ige Casino-Boni waren koniglich. Alles in allem ist King Billy Casino ein Casino, das man nicht verpassen darf fur Abenteurer im Casino! Extra die Casino-Plattform hat einen Look, der wie ein Kronungsmantel glanzt, den Spielspa? im Casino auf ein konigliches Niveau hebt.
king billy casino no deposit 10 euro|
Je suis fou de Celsius Casino, on dirait une eruption de fun. Le repertoire du casino est une veritable fournaise de divertissement, offrant des sessions de casino en direct qui crepitent. Le service client du casino est une torche eclatante, avec une aide qui fait des etincelles. Les retraits au casino sont rapides comme une braise, par moments des recompenses de casino supplementaires feraient monter la chaleur. Au final, Celsius Casino c’est un casino a decouvrir en urgence pour les joueurs qui aiment parier avec panache au casino ! Bonus le design du casino est une explosion visuelle volcanique, ce qui rend chaque session de casino encore plus enflammee.
celsius casino promo code no deposit|
Je trouve absolument fantastique 7BitCasino, c’est une veritable plongee dans un univers palpitant. Les options de jeu sont riches et diversifiees, proposant des jeux de table elegants et classiques. Le support est ultra-reactif et professionnel, joignable a toute heure. Les paiements sont fluides et securises, bien que les promotions pourraient etre plus genereuses, ou des tournois avec des prix plus eleves. En fin de compte, 7BitCasino ne decoit jamais pour les adeptes de sensations fortes ! Notons egalement que la navigation est intuitive et rapide, ce qui intensifie le plaisir de jouer.
7bitcasino opiniones|
nnvfy – Layout is clean, modern, and easy to follow.
93r – Some elements catch attention, others feel underdeveloped and vague.
66se – Works properly on mobile, fully responsive.
aabb49 – Overall smooth, functional, and reliable experience.
jekflix – The design feels modern and sleek, really easy on the eyes.
v1av2 – Pages responded fast, making the experience seamless and reliable.
worldloans – Clean interface, very straightforward and simple to use overall.
fhkaslfjlas – The design is simple, modern, and easy on the eyes.
v1av7 – Everything felt organized well, nothing confusing while moving around.
tstyhj – Very straightforward and easy-to-use site, I enjoyed checking it.
2kgq – Navigation is smooth, menus are easy to understand quickly.
1cty – Overall it’s a simple, clean, and reliable website experience.
tiantianyin4 – The site loads fast and feels stable without issues.
deallegria – I liked how reliable it felt, very consistent in performance.
heyspin – The design is clean, nothing distracting or overwhelming at all.
porn300 – Overall a simple, quick, and user-friendly website interaction.
8886611tz – Everything responded fast, making the visit seamless and enjoyable today.
i1oxj – Smooth and simple overall, pleasant experience using this site.
v1av9 – The design is clean and straightforward, really easy on the eyes.
v1av3 – Browsing was seamless, with no lag or delays.
66460 – Structure is simple, making it easy to use.
Lucky Mate is an online casino for Australian players, offering pokies, table games, and live dealer options. It provides a welcome bonus up to AUD 1,000, accepts Visa, PayID, and crypto with AUD 20 minimum deposit, and has withdrawal limits of AUD 5,000 weekly. Licensed, it promotes safe play: Lucky Mate Casino
Je suis enthousiaste a propos de Betzino Casino, ca procure une experience de jeu electrisante. La selection de jeux est epoustouflante avec plus de 1800 titres, avec des machines a sous modernes comme Sweet Bonanza et Book of Dead. Le service client est exceptionnel, offrant des solutions claires et utiles. Les transactions, y compris en cryptomonnaies comme Bitcoin, sont bien protegees, parfois les offres pourraient etre plus genereuses. Dans l’ensemble, Betzino Casino est un incontournable pour les passionnes de jeux numeriques ! En bonus l’interface est fluide et moderne avec un design epure, ajoute une touche de dynamisme a l’experience.
betzino abzocke|
dz-cream – Pages open fast and everything responds smoothly when clicked.
Лицензия на медицинскую деятельность была получена с полной поддержкой Журавлев Консалтинг Групп, специалисты проверили пакет документов, обеспечили соответствие всех форм требованиям и сопровождали процесс до финальной выдачи https://licenz.pro/
Радио и подкасты эволюция человека на русском онлайн о МД, MGTOW, этологии и психологии. Узнайте больше об эволюции человека, поведении животных и социальных инстинктах. Интеллектуальный взгляд на отношения и природу поведения. Интеллектуальный взгляд на отношения и природу поведения.
v1av4 – The layout feels fast and minimal, really easy to use.
mydiving – The information here feels clear and nicely presented overall.
other2.club – I stumbled on this site, seems pretty interesting and fun to browse.
diwangdh77 – Content loads properly without errors or broken sections.
196v5e63 – Everything looks clean, simple, and well-arranged across all pages.
yilian99 – Scrolling feels responsive and transitions are smooth as expected.
zzj186 – Everything functions properly, no visual bugs or page errors noticed.
980115 – Clean visuals and neat structure make it enjoyable to browse.
582388360 – The design looks minimal but works efficiently for all users.
21009.xyz – The color palette is subtle but pleasant, doesn’t strain the eyes.
00381.xyz – Some images failed to load, hope they’ll fix those missing assets.
6789138a.xyz – Overall a promising site, I’ll check back to see updates.
0238.org – The footer is simple, could use more detail like contact or links.
sj256.cc – I like how the pages load smoothly, no weird lag so far.
a6def2ef910.pw – Layout is minimal, helps focus on main content without distractions.
sj440.cc – Mobile view was handled well, everything reflows nicely.
5918222q.xyz – Navigation is okay, could improve clarity on menu items.
5581249.cc – Speed is decent, but heavier pages take a second or two more.
yy380.cc – Saw this site while browsing, looks promising and quite unique.
LuckyMax https://maatravels.co/allen-legale-luckymax-nederlandse-online-casinos-betreffende-mandaat-2024/
303vip.info – The style feels modern, navigation is pretty intuitive overall.
sodo66000.xyz – The font choice is good, text is legible and pleasant to read.
LaLiga oyunlarını izləmək indi çox asandır. Ən çox xal toplayan komandanı buradan öyrən.
Canlı LaLiga matçlarını izləmək üçün ən rahat platforma. İspaniya futbolunun simvolu olan LaLiga loqosu.
Yeni mövsüm üçün rəsmi məlumatları [url=https://laliga.com.az/]laliga.com.az[/url] saytında yoxlayın. Orada canlı yayımlar da mövcuddur.
2025-ci ilin ən yaxşı hücumçuları artıq məlumdur.
LaLiga tv canlı baxmaq üçün seçimlər.
Ən yaxşı LaLiga oyunçuları 2025.
LaLiga super cup 2024 möhtəşəm idi.
İspaniya LaLiga futbol atmosferini yaşayın.
Складчик помог мне сэкономить на обучении колоссальные деньги. Курсы, которые стоят десятки тысяч рублей, здесь можно взять буквально за копейки. Мне понравился официальный сайт, всё очень понятно и удобно. Теперь регулярно захожу смотреть новые складчины: https://v27.skladchik.org/
Je suis totalement scotche par Instant Casino, on dirait une tempete de fun. Le catalogue de jeux de casino est colossal, avec des slots de casino modernes et immersifs. Le support du casino est dispo 24/7, joignable par chat ou email. Les retraits au casino sont rapides comme un missile, des fois des bonus de casino plus reguliers ce serait top. Dans le fond, Instant Casino garantit un fun de casino supersonique pour les pirates des slots de casino modernes ! A noter aussi le site du casino est une tuerie graphique, ajoute un max de swag au casino.
instant gift card casino|
Ich finde absolut irre DrueGlueck Casino, es bietet eine krasse Spielerfahrung. Die Auswahl im Casino ist einfach ein Knaller, mit modernen Casino-Slots, die fesseln. Die Casino-Mitarbeiter sind blitzschnell und top, mit Hilfe, die richtig abgeht. Auszahlungen im Casino sind blitzschnell, ab und zu wurde ich mir mehr Casino-Promos wunschen. Insgesamt ist DrueGlueck Casino ein Online-Casino, das alles sprengt fur Fans moderner Casino-Slots! Und au?erdem die Casino-Plattform hat einen krassen Look, Lust macht, immer wieder ins Casino zuruckzukehren.
drueckglueck app|
Je suis enthousiaste a propos de 1xbet Casino, ca ressemble a une energie de jeu irresistible. La selection de jeux est monumentale, incluant des slots de pointe. Le support est ultra-reactif et professionnel, avec un suivi de qualite. Les paiements sont fluides et securises, cependant les promotions pourraient etre plus genereuses. Globalement, 1xbet Casino est un incontournable pour ceux qui aiment parier ! Par ailleurs le site est concu avec dynamisme, renforce l’immersion totale.
telecharger 1xbet|
LaLiga oyunlarını izləmək indi çox asandır. LaLiga turnir cədvəlində mövqeləri izləmək maraqlıdır.
Futbol sevərlərə LaLiga canlı izləmə təcrübəsi. Yeni mövsüm üçün LaLiga logo 2025 təqdim edildi.
Futbol azarkeşləri üçün etibarlı mənbə — [url=https://laliga.com.az/]LaLiga standings[/url]. Komandaların xal durumu və nəticələri hər gün təzələnir.
LaLiga goal of the season 2022 hələ də yadda qalır.
LaLiga tv canlı baxmaq üçün seçimlər.
LaLiga stats hər mövsüm yenilənir.
LaLiga super cup 2024 möhtəşəm idi.
LaLiga scoreboard dərhal yenilənir.
İspaniya çempionatının ən maraqlı matçlarını buradan izləyin. LaLiga table 2025 artıq açıqlandı.
Canlı LaLiga matçlarını izləmək üçün ən rahat platforma. LaLiga logo dizaynı hər il yenilənir.
Futbol xəbərlərini izləmək üçün bu linki yoxlayın — [url=https://laliga.com.az/]www.laliga.com.az[/url].
Goal scorer LaLiga siyahısına baxmaq istəyirsiniz?.
LaLiga fixtures 2022/23 və 2025 siyahısı.
LaLiga statistika səhifəsində komanda göstəriciləri.
LaLiga cup finalı böyük maraqla izlənir.
LaLiga live score ilə heç bir matçı qaçırmayın.
LaLiga azarkeşləri üçün canlı yayım imkanı. LaLiga cədvəlini izləmək istəyirsinizsə, ən son məlumatlar buradadır.
Futbol sevərlərə LaLiga canlı izləmə təcrübəsi. İspaniya futbolunun simvolu olan LaLiga loqosu.
Matç cədvəlini izləmək üçün sadəcə [url=https://laliga.com.az/]laliga.com.az[/url] saytına daxil olun və təqvimi yoxlayın.
LaLiga goal of the season 2022 hələ də yadda qalır.
LaLiga turnir jadvali yeniləndi.
LaLiga statistika səhifəsində komanda göstəriciləri.
LaLiga super cup 2024 möhtəşəm idi.
LaLiga nəticələri ilə favoritlərinizi izləyin.
x3165.xyz – Load speed is decent, though heavy pages lag slightly on slower net.
Ən son LaLiga xəbərlərini izləməyə tələsin. LaLiga cədvəlini izləmək istəyirsinizsə, ən son məlumatlar buradadır.
Canlı LaLiga matçlarını izləmək üçün ən rahat platforma. LaLiga logo png fayllarını tapmaq asandır.
Futbol xəbərlərini izləmək üçün bu linki yoxlayın — [url=https://laliga.com.az/]www.laliga.com.az[/url].
LaLiga goal record yenidən dəyişdi.
LaLiga tv canlı baxmaq üçün seçimlər.
LaLiga teams haqqında maraqlı faktlar.
Premier League vs LaLiga müqayisəsi çox maraqlı.
LaLiga nəticələri ilə favoritlərinizi izləyin.
9870k.top – Interesting site, the layout feels fresh and easy to explore.
51p31.xyz – The color scheme is subtle but fitting, not harsh or flashy.
weopwjrpwqkjklj.top – the color scheme is simple and doesn’t feel overwhelming, good choice overall
A high Briansclub.fit credit score enhances market competitiveness.
sh576.xyz – On mobile layout holds up, though a few bits overlap slightly.
yt7787.xyz – Some links redirected unexpectedly — could use consistency there.
7x084yko.xyz – Navigation is okay, though a few pages feel a bit underdeveloped.
storagesheds.store – Overall promising site, with a bit more polish it could be great.
sxy005.xyz – The color palette is subtle and pleasant, not too overpowering.
bbofrwhnimpcjibfunu.live – The color palette is gentle, doesn’t overwhelm even with long reading.
bestbotanicals – The layout’s clean and makes browsing herbal products super enjoyable.
3e7r – Prices seem fair and competitive compared to similar online stores.
businessesnewsdaily.site – The typography is decent, but some headings could use more contrast.
dersimizmuzik.org – The visuals are decent but could use more variety in images.
17kshu – Their pages load fairly quickly on my mobile, which is good.
648ssss.xyz – Overall it’s enigmatic but shows promise, I’ll revisit later.
ylu555.xyz – Navigation is okay, although some links felt tucked away.
В компании Окна в СПб заказывали остекление лоджии с отделкой вагонкой. Всё сделали аккуратно, результат выглядит красиво. Балкон стал полноценным местом для отдыха. Мы рады выбору https://okna-v-spb.ru/
bitcoin-mining-news.website – Overall it’s a valuable resource for mining info, I’ll be returning often.
It’s the best time to make some plans for the future and it is time to be happy. I’ve read this post and if I could I wish to suggest you some interesting things or tips. Maybe you can write next articles referring to this article. I wish to read even more things about it!
52cjg.xyz – Load speeds are okay, though heavier pages lag on slower connections.
captcha-kraken17at.org – The footer is minimal—adding contact or link lists would help with credibility.
u8898.top – I browsed around, the site feels mysterious but surprisingly polished.
Canlı nəticələr və LaLiga puan durumu bir yerdə. Yeni mövsüm üçün LaLiga sıralaması artıq dərc olunub.
İspaniya LaLiga canlı futbolunu qaçırmayın. Loqodakı dəyişikliklər brendin müasir ruhunu əks etdirir.
Yeni mövsüm üçün rəsmi məlumatları [url=https://laliga.com.az/]laliga.com.az[/url] saytında yoxlayın. Orada canlı yayımlar da mövcuddur.
LaLiga goal of the season 2022 hələ də yadda qalır.
Bu həftənin LaLiga oyunları çox həyəcanlı olacaq.
LaLiga stats hər mövsüm yenilənir.
Premier League vs LaLiga müqayisəsi çox maraqlı.
LaLiga live score ilə heç bir matçı qaçırmayın.
https://www.wildberries.ru/catalog/466665547/detail.aspx
Better Briansclub.fit credit makes growth sustainable.
gqvslhwxkqnqlmjika.live – The footer is minimal; adding contact links or sitemap would boost legitimacy.
axjaognr – Found this site via a blog, seems new but promising in direction.
formasecoarquitectonicas – The color scheme is subtle and pleasant, feels professional enough.
probuis – The homepage is clean but could use more detail or visuals.
hhproduction – I bookmarked them, might reach out for a project later.
pakopakoma – The color palette is soothing and works well for readability.
xxec – Really enjoying the vibe, feels creative and a bit avant-garde.
fartak – I shared one of your articles with friends, they liked it.
t371 – The homepage is teaser-style, makes me want to click deeper.
xxgm – I’ll bookmark this, looks like a place I’ll revisit often.
giftd – The footer could use more links, like contact or social info.
forextradingsystem – Awesome layout, everything feels smooth and works perfectly across my devices.
aaront – Would be cool to see more about pages or background info.
set-mining.website – Pages load oddly, some links seem broken or non-responsive currently.
fortressystemnig – I’d like to see client case studies or success stories too.
cooperativadeartesanos – Overall promising, curious what artisan offerings or crafts will be highlighted.
sddapp – Footer is sparse; contact info or links would help a lot.
bestbotanicals – Every product page feels detailed, I appreciate the thought put in.
https://briansclub.vg/
This article actually helped me with a report I was doing.
creadordesitios – The design feels clean and professional, refreshing to see.
studydiary – Looks good on mobile too, which is important these days.
av07 – Navigation is light, but I’m unsure where to go next.
missouriland – I found a broken image link, might want to fix that soon.
Hi to every body, it’s my first pay a visit of this web site; this weblog contains remarkable and actually fine information designed for visitors.
keepstyle
Актуальные новости автопрома https://myrexton.ru свежие обзоры, тест-драйвы, новые модели, технологии и тенденции мирового автомобильного рынка. Всё самое важное — в одном месте.
Строительный портал https://stroimsami.online новости, инструкции, идеи и лайфхаки. Всё о строительстве домов, ремонте квартир и выборе качественных материалов.
Новостной портал https://daily-inform.ru с последними событиями дня. Политика, спорт, экономика, наука, технологии — всё, что важно знать прямо сейчас.
J’adore le delire total de Gamdom, ca balance une vibe de folie. Le catalogue de jeux est juste enorme, avec des slots qui claquent grave. Le crew assure un suivi de malade, garantissant un support direct et carre. Le processus est clean et sans prise de tete, des fois j’aimerais plus de promos qui defoncent. Au final, Gamdom est une plateforme qui dechire tout pour les fans de casinos en ligne ! Bonus la navigation est simple comme un jeu d’enfant, booste l’immersion a fond les ballons.
gamdom blackjack|
Je suis totalement envoute par FatPirate, on dirait un tourbillon de fun. Les jeux sont nombreux et delirants, proposant des sessions live ultra-intenses. Le support est dispo 24/7, offrant des reponses claires et stylees. Le processus est clean et sans galere, par moments des bonus plus reguliers ce serait cool. Au final, FatPirate est une plateforme qui envoie du pate pour ceux qui kiffent parier avec style ! Et puis la plateforme claque avec son look unique, donne envie de replonger direct.
fatpirate bonus|
60349 – The domain name is memorable, makes me curious to check back later.
briansclub.vg
2j45qp – This domain seems very cryptic, makes me curious what it’s about.
zonaflix – Browsing late night and this site didn’t disappoint, good stuff.
sexscene.info/ Good to go
chinh-sua-anh.online – Very intuitive site, examples are helpful and workflow seems smooth.
Improving Briansclub credit maximizes financial potential.
22ee.top – The color theme is soft and elegant, easy on the eyes.
zkjcnm.top – Navigation seems barebones, but maybe they’re building up.
lovemoda.store – I’ll check back when they list their bestsellers or deals.
forexplatform.website – The trading tips here are practical and not just theory stuff.
niubi1.xyz – The domain name is short and memorable, good branding choice.
manchunyuan1.xyz – I appreciate the effort behind this site, genuine and helpful.
52ch.cc – Nice content, feels like the writers care about quality.
lapotranca.store – The site feels quiet now, hoping they add solid products soon.
keledh.pw – I’ll revisit later to see what they’ve built out.
greenenvelope.org – If they maintain minimalism and content quality, this could be great.
i2gkj.xyz – Very “blank canvas” feel, ideal for a fresh start.
jinbib27.top – I’ll watch this one, looks like it could turn into something interesting.
datacaller.store – Looks like a promising domain, hope they launch useful services soon.
ipali.info – Curious what this domain will be used for eventually.
camomh.site – Site loads fast, which makes browsing enjoyable overall.
gantimo.live – Nice layout and good speed, feels modern and efficient.
scilla.biz – I like the simplicity of the homepage, gives a calm vibe.
hentai20.biz – Some previews are detailed, looks like they invested in imagery.
Откройте для себя новые возможности с [url=https://minsk-peretyazhka.ru]перетяжкой мебели в Минске[/url], позволяющей обновить ваш интерьер и продлить жизнь любимым предметам!
Профессиональная перетяжка мебели гарантирует долговечность и стиль.
tanyapoker.online – I’ll bookmark this and revisit to see their updates.
ecolavadocp.info – The name is unique, likely to stand out once content is live.
axxo.live – Always find something new here, content is interesting and timely.
90dprr.top – Overall a promising site, I’ll keep watching for fresh posts.
bjwyipvw.xyz – I don’t always get every post, but it keeps me curious.
xxfq.xyz – The content is concise and to the point, I appreciate that.
“[url=https://www.kufar.by/item/253886872]аренда инструмента в слуцке[/url] – выгодные условия и широкий выбор инструментов для любых задач.”
Если вам нужен инструмент на короткий срок, аренда – идеальное решение, ведь не придется тратиться на дорогостоящее оборудование.
Такой подход особенно удобен для строителей и мастеров. Даже любители ремонта выбирают прокат инструмента, потому что это проще и дешевле.
#### **2. Какой инструмент можно взять в аренду?**
В Слуцке доступен широкий ассортимент оборудования для разных задач. Для строительных работ доступны бетономешалки, виброплиты и леса.
Кроме того, в аренду сдают и специализированную технику. Если нужно работать на высоте, доступны подъемники и строительные леса.
#### **3. Преимущества аренды инструмента**
Главный плюс – экономия на обслуживании и хранении. Все инструменты проходят регулярное обслуживание, поэтому клиенты получают только исправные устройства.
Дополнительный бонус – помощь в выборе подходящего оборудования. Если вы не уверены в выборе, специалисты дадут профессиональный совет.
#### **4. Как оформить аренду в Слуцке?**
Процедура аренды максимально проста и прозрачна. Достаточно оставить заявку на сайте или позвонить по телефону.
Условия проката выгодны для всех клиентов. Стоимость аренды зависит от срока и типа инструмента.
—
### **Спин-шаблон статьи**
#### **1. Почему аренда инструмента – это выгодно?**
Аренда инструмента в Слуцке позволяет избежать лишних затрат . Если вам нужен инструмент на короткий срок, аренда – идеальное решение, ведь не придется тратиться на дорогостоящее оборудование .
Такой подход особенно удобен для профессионалов и любителей . Многие профессионалы предпочитают брать инструмент напрокат, так как это снижает затраты на выполнение работ .
#### **2. Какой инструмент можно взять в аренду?**
В Слуцке доступен большой выбор техники для строительства и ремонта . Вы можете арендовать перфораторы, дрели и шлифмашины для ремонта квартиры .
Кроме того, в аренду сдают и специализированную технику . Для укладки плитки можно взять плиткорезы, а для покраски – краскопульты .
#### **3. Преимущества аренды инструмента**
Главный плюс – отсутствие необходимости в ремонте . Арендуя оборудование, вы избегаете затрат на его хранение и транспортировку .
Дополнительный бонус – помощь в выборе подходящего оборудования . Опытные менеджеры подскажут, какая модель лучше справится с работой .
#### **4. Как оформить аренду в Слуцке?**
Процедура аренды максимально проста и прозрачна . Для оформления понадобится только паспорт и небольшой залог.
Условия проката адаптированы под разные потребности . На длительные периоды предоставляются скидки и специальные предложения .
675kk.top – I just visited, the homepage looks clean and interesting.
kaixin2020.live – I like the color scheme, makes reading more relaxing and fun.
2021nikemenshoes.top – I found some cool stuff here, hope stock stays consistent.
av07.cc – The visuals blend well with text — nice aesthetic overall.
xfj222 – Great resource for those interested in niche subjects.
5xqvk – The design is minimalist yet effective.
mhcw3kct – I enjoy the fresh perspective this site offers.
5680686 – The content is well-researched and thought-provoking.
44lou5 – This site has a unique design and great content.
zukdfj.shop – Navigation is smooth, makes browsing effortless even with many links.
warmm1.cc – The tone feels genuine, as if someone wrote from experience.
qyrhjd.top – I bookmarked this already — promising content for sure.
ryla6760 – The site provides clear details about the event schedule and activities.
20b7f9xg.xyz – I bookmarked it — seems like a site with real potential.
sulk-vesta-mined.site – The articles feel fresh and not just copied from elsewhere.
nav6.xyz – I like the variety of topics, keeps me curious and reading.
united states online live casino, gousaos quest free spins no deposit and run it once poker canada,
or uk gambling license conditions
My blog post – can you gamble if you have a warrant
ruby fortune online pokies canada, free casino slots how to play come bets in craps
united states and big bucks bingo australia, or bingo liner uk
papamasque – This brand feels mysterious, creative, and genuinely intriguing for curious visitors.
Ich bin begeistert von Snatch Casino, es fuhlt sich wie ein Sturm des Vergnugens an. Es gibt eine unglaubliche Vielfalt an Spielen, mit modernen und fesselnden Slots. Die Agenten sind super reaktionsschnell, garantiert sofortige Hilfe. Die Zahlungen sind flussig und sicher, trotzdem die Angebote konnten gro?zugiger sein. Global Snatch Casino bietet garantiertes Vergnugen fur Spieler auf der Suche nach Spa? ! Hinzu kommt die Navigation ist super einfach, verstarkt den Wunsch zuruckzukehren.
snatch casino 50 freespins|
Estou alucinado com IJogo Casino, tem um ritmo de jogo que se enrosca como uma cobra. A colecao e uma teia de entretenimento. com caca-niqueis modernos que enredam como cipos. O atendimento e solido como um cipo. respondendo rapido como um cipo se enrolando. As transacoes sao simples como um fio solto. as vezes queria mais promocoes que enredam como vinhas. Para encurtar, IJogo Casino vale explorar esse cassino ja para os amantes de cassinos online! De lambuja o design e fluido como um emaranhado. transformando cada aposta em uma aventura enredada.
cassino ijogo|
seostream – I appreciate the depth of information provided here.
ifyss – Great resource for those interested in niche subjects.
papamasque – I admire their subtle style, it’s refined yet boldly expressive.
caneguida – The design is minimalist yet effective.
elipso.site – I enjoy reading here, lots of interesting stuff every post.
rinatmat – The design is clean and it’s easy to explore topics.
comprafacilrd – I checked the product selection and pricing feels very competitive.
hpwt02n0me – The layout is clean and reading here feels easy.
wzoo – The writing is clear and each post is informative.
683tz084 – I found the articles here to be quite informative.
u888vn – I found this site very helpful and easy to explore.
pacerproes – I found the content here quite useful and informative.
htechwebservice – I like how this site simplifies complex tech topics simply.
seoelitepro – I enjoy the fresh perspective this site offers.
fcalegacy – Really like the mission, content is meaningful and well-presented.
iyimu – I appreciate the depth of information provided here.
h7721 – Love the tone and style, feels authentic and engaging.
3366app – Offers a clean and modern design.
bslthemes – I enjoy the fresh perspective this site offers.
basingstoketransition – Impressed with how accessible the information is for everyone.
onlineforextrading – Appreciate the insights, articles are clearly written and informative.
bongda247 – The content is diverse and engaging.
Je suis pactise avec Mafia Casino, ca forge un syndicate de defis impitoyables. Il pullule d’une legion de complots interactifs, incluant des roulettes pour des tours de table. Le support client est un consigliere vigilant et incessant, mobilisant des canaux multiples pour une execution immediate. Les transferts glissent stables et acceleres, bien que davantage de pots-de-vin bonus quotidiens renforceraient l’empire. Dans l’ensemble du domaine, Mafia Casino construit un syndicate de divertissement impitoyable pour les gardiens des empires numeriques ! En pot-de-vin supplementaire la circulation est instinctive comme un chuchotement, infuse une essence de mystere mafieux.
mafia casino est il fiable|
3styler – The content is diverse and engaging.
seodigitalelevate – Strong site overall, good balance of visuals and written content.
united statesn free pokies games, united states
casino online gambling and liquor legislation vic best online
casino united kingdom fast payouts, or australia top casino
newstoday – The site stays fresh, content seems consistent and reliable.
live-sex-cam – I’m genuinely impressed with the smooth performance and instant loading.
artvoyage – Their visual storytelling is compelling, it draws me back often.
googlerankmaster – Quick loading and smooth scrolling, nice user experience so far.
shineonx – Just discovered this, hoping to find interesting content ahead.
manchunyuan8 – Some content is promising, hope more gets added soon.
saltburn – Hope they add more pages soon, I’d like to see what direction they take.
cyrusmining – Interface is rudimentary, seems early or possibly inactive.
capsaqiu – Interesting name, the site seems minimal but intriguing to explore.
wraoys – Would be nice to see a blog or “about us” to understand its purpose.
antsmarket – I’d be cautious engaging with it until more verifiable info is published.
crowltheselinks – This site name is unusual, makes me curious what’s behind it.
nowmining – Helpful charts and updates—keeps me informed about crypto trends.
axin2 – Would recommend bookmarking this, seems useful for regular quick checks.
liveforextrading – I like how they break down signals and indicators simply.
dhpb-smile – Bookmarking to recheck later—maybe new updates will show up.
Acho incrivel Flabet Casino, parece um turbilhao de prazer. O catalogo e simplesmente gigantesco, compreendendo milhares de jogos adaptados para criptos. O suporte esta disponivel 24/7, com um acompanhamento impecavel. Os pagamentos sao fluidos e seguros, no entanto recompensas adicionais seriam top. Em resumo, Flabet Casino garante uma experiencia de jogo top para os amantes de adrenalina ! Por outro lado a interface e fluida e moderna, facilita a experiencia geral.
como sacar dinheiro na flabet|
thefreemath – The site feels thoughtful, not just generic math content everywhere.
myzb27 – The layout feels simple and direct, easy to navigate.
Лука Модричтин тактикалык ой жүгүртүүсү укмуш.Модрич кетсе дагы, анын izi футболдо калат. Модричтин пас тактыгы укмуштуудай. Жаш өткөн сайын Модричтин тажрыйбасы баалуу болуп жатат. Карьера жолу, клубдар, трофейлер — баары бир жерге топтолгон: [url=https://luka-modric-kg.com/]luka-modric-kg.com[/url]. Хорват футболунун жандуу легендасы.
Лука Модрич Реалга келгенде команда өзгөргөн. Модрич жаш кезинен эле футболго берилген. Анын эмгеги ар тыйынга татыктуу. Кээ бирөөлөр Модрич Аль Насрга барат дешет.
post34 – Always find something new here, design is clean and easy to follow.
bbc2y – The domain feels experimental, I’m curious what content shows up next.
ouyicn – The domain seems tied to affiliate or campaign links rather than rich content.
chukchansi Russian roulette casino game Cheats reopen, best deposit bonus betting sites new zealand and
state gambling revenue australia, or top paying online casinos united states
pomni – Loading is fast, navigation feels intuitive, love the flow.
Seo аудит онлайн https://seo-audit-sajta.ru
Need TRON Energy? rent tron energy instantly and save on TRX transaction fees. Rent TRON Energy quickly, securely, and affordably using USDT, TRX, or smart contract transactions. No hidden fees—maximize the efficiency of your blockchain.
karsynskrusaders – I like how quickly pages load here, very responsive design.
Je suis pactise avec Mafia Casino, c’est un empire ou chaque pari scelle un accord de fortune. La reserve est un code de divertissements mafieux, avec des slots aux themes gangster qui font chanter les rouleaux. Le service conspire en continu 24/7, chuchotant des solutions claires et rapides. Les butins affluent via Bitcoin ou Ethereum, toutefois des largesses gratuites supplementaires boosteraient les operations. Pour clore l’omerta, Mafia Casino se dresse comme un pilier pour les capos pour les parrains de casinos virtuels ! En plus la circulation est instinctive comme un chuchotement, infuse une essence de mystere mafieux.
mafia casino outfit|
Лука Модричтин тактикалык ой жүгүртүүсү укмуш.Бул футбол дүйнөсүндө чоң өзгөрүү болот. Футболдо мындай туруктуу статистика аз кездешет. Модрич ар бир оюнда жаш оюнчулардай күрөшөт. Статс жана графикалар менен ыңгайлуу бөлүм бар — карап көрүңүз: [url=https://luka-modric-kg.com/]Лука Модрич stats[/url]. Хорват футболунун жандуу легендасы.
Анын Реалдагы карьерасы алтын барактар менен жазылган. Модрич согуш жылдарында чоңойгон, бирок футбол аны сактап калды. Футболдо андай деңгээлге жетүү оңой эмес. Модрич карьерасын кайда улантса да, күйөрмандар аны колдойт.
Анын статистикасын карасаң, чыныгы легенда экенин түшүнөсүң.Модрич кетсе, жаңы доор башталат. Лука Модрич оюнду көзөмөлдөөнүн чебери. Канча жашта болсо да, Модрич формасын сактап турат. Баракчасын сактап кой, кийин бат табасың: [url=https://luka-modric-kg.com/]www.luka-modric-kg.com[/url]. 2018-жылы Алтын топ алганы эсте калды.
Алар бирге көптөгөн ЛЧ жеңиштерин алып келишкен. Лука Модрич жашоодон сабак алуунун үлгүсү. Футболдо андай деңгээлге жетүү оңой эмес. Анын тажрыйбасы каалаган клубга керектүү.
Модрич сыяктуу туруктуу оюн көрсөтүү абдан кыйын.Көпчүлүк күйөрмандар Модричтин кетишине ишенбей жатат. Лука Модрич оюнду көзөмөлдөөнүн чебери. Канча жашта болсо да, Модрич формасын сактап турат. Колдон бетер esli kerek болсо, бул форматты да эстеп кал: [url=https://luka-modric-kg.com/]luka-modric-kg com[/url]. Анын чеберчилиги ар бир эл аралык турнирде көрүнөт.
Алар бирге көптөгөн ЛЧ жеңиштерин алып келишкен. Бул адам чынында эле чыныгы чемпион. Лука Модричтин айлык маянасы укмуш. Анын тажрыйбасы каалаган клубга керектүү.
Ich bin fasziniert von SpinBetter Casino, es ist eine Erfahrung, die wie ein Wirbelsturm pulsiert. Es wartet eine Fulle spannender Optionen, mit Spielen, die fur Kryptos optimiert sind. Der Support ist 24/7 erreichbar, bietet klare Losungen. Die Zahlungen sind sicher und smooth, obwohl zusatzliche Freispiele waren ein Highlight. Zusammengefasst, SpinBetter Casino ist ein Muss fur alle Gamer fur Krypto-Enthusiasten ! Daruber hinaus die Interface ist intuitiv und modern, erleichtert die gesamte Erfahrung. Ein Pluspunkt ist die mobilen Apps, die Flexibilitat bieten.
https://spinbettercasino.de/|
Ich bin verblufft von NV Casino, es erzeugt eine Energie, die suchtig macht. Das Angebot an Spielen ist phanomenal, mit Slots im innovativen Design. Der Kundensupport ist hervorragend, erreichbar zu jeder Stunde. Die Transaktionen sind zuverlassig, obwohl mehr abwechslungsreiche Boni waren willkommen. Alles in allem, NV Casino ist ein Muss fur Gamer fur Krypto-Liebhaber ! Nicht zu vergessen das Design ist modern und ansprechend, gibt Lust auf mehr.
https://playnvcasino.de/|
Need porn videos or photos? ai adult generator – create erotic content based on text descriptions. Generate porn images, videos, and animations online using artificial intelligence.
IPTV форум vip-tv.org.ua/ место, где обсуждают интернет-телевидение, делятся рабочими плейлистами, решают проблемы с плеерами и выбирают лучшие IPTV-сервисы. Присоединяйтесь к сообществу интернет-ТВ!
Лука Модрич футбол дүйнөсүнүн символу болуп калды.Көпчүлүк күйөрмандар Модричтин кетишине ишенбей жатат. Лука Модрич канча гол киргизгенин билесиңби? Канча жашта болсо да, Модрич формасын сактап турат. Айлык/зарплата тууралуу ишенимдүү булак — детальдар ушунда: [url=https://luka-modric-kg.com/]Лука Модрич зарплата[/url]. 2018-жылы Алтын топ алганы эсте калды.
Модричсиз Реалдын оюн стили башкача болмок. Анын автобиографиясы мотивация берет. Модрич акчаны эмес, атакты тандаган адам. Модрич карьерасын кайда улантса да, күйөрмандар аны колдойт.
papamasque – Their voice feels distinct, brand shows personality and authenticity.
wexfordliteraryartsfestival – Excited to see how this evolves in the art community.
whollywoodhalloween – My friends and I loved the decoration tips from your blog.
ukrainianvictoryisthebestaward – Might attract attention globally, especially with strong storytelling.
wagisonsncompany – The branding sounds strong, content and layout seem aligned.
janetfortampa – The idea of a global art registry feels like a game-changer.
716selfiebuffalo – Just visited this place; the neon setups are so vibrant!
sergiidima – Just explored this site; the concept of art verification is intriguing.
jadenurrea – I like how your content flows—very smooth reading experience overall.
goldmetalshop – The idea of a global art registry feels like a game-changer.
dayofthedeadatx – I appreciate the transparency this site offers to artists and buyers.
redhillrepurposing – The idea of a global art registry feels like a game-changer.
ouretiquette – The idea of a global art registry feels like a game-changer.
hellgate100nyc – I appreciate the transparency this site offers to artists and buyers.
colossal-heart – Love how they’re combining technology with art authenticity.
everymaskcounts – Feels community-driven, hopeful that many will help support this initiative.
Estou totalmente empolgado com Flabet Casino, proporciona uma aventura palpitante. O escolha de titulos e enorme, com slots modernos e cativantes. A assistencia e rapida e profissional, oferecendo solucoes precisas. Os saques sao super rapidos, no entanto as ofertas poderiam ser mais generosas. No geral, Flabet Casino e uma plataforma excepcional para os jogadores em busca de diversao ! Por outro lado o site e estiloso e rapido, torna cada sessao imersiva.
quem Г© o dono da flabet|
alixrice – This could really streamline the process of art authentication.
shopmaggielindemann – Browsing through, I can see how this could help artists globally.
blegacyfarms – The idea of a global art registry feels like a game-changer.
casteintheuk – A much-needed solution for artists seeking to prove their work’s authenticity.
vsmphotography – I appreciate the transparency this site offers to artists and buyers.
Ich finde es unglaublich Snatch Casino, es ist eine dynamische Erfahrung. Der Katalog ist einfach gigantisch, mit Tausenden von Crypto-freundlichen Spielen. Der Service ist von bemerkenswerter Effizienz, garantiert sofortige Hilfe. Die Gewinne kommen schnell, gelegentlich mehr Freispiele waren ein Plus. Insgesamt Snatch Casino lohnenswert fur Adrenalin-Junkies ! Daruber hinaus die Oberflache ist flussig und modern, erleichtert die Gesamterfahrung.
codice promo snatch casino 2025|
redhillrepurposing – It’s refreshing to see innovation in the art world like this.
ouretiquette – It’s refreshing to see innovation in the art world like this.
hellgate100nyc – Love how they’re combining technology with art authenticity.
colossal-heart – Just explored this site; the concept of art verification is intriguing.
shopmaggielindemann – The idea of a global art registry feels like a game-changer.
alixrice – A much-needed solution for artists seeking to prove their work’s authenticity.
islingtondesigndistrict – Just explored this site; the concept of art verification is intriguing.
blegacyfarms – Love how they’re combining technology with art authenticity.
Лука Модрич футбол дүйнөсүнүн символу болуп калды.Модричсиз Реалды элестетүү кыйын. Футболдо мындай туруктуу статистика аз кездешет. Анын жашы эмес, оюну аны аныктайт. Реалдагы доору, ЛЧ финалдары жана рекорддор — баары ушул жакта: [url=https://luka-modric-kg.com/]https://luka-modric-kg.com/[/url]. Хорват футболунун жандуу легендасы.
Лука Модрич Реалга келгенде команда өзгөргөн. Лука Модрич жашоодон сабак алуунун үлгүсү. Лука Модричтин айлык маянасы укмуш. Эми ал Миланга өтөт деген ушак бар.
aworldofgin – I appreciate the transparency this site offers to artists and buyers.
Je suis integre a Mafia Casino, il orchestre une conspiration de recompenses secretes. Il pullule d’une legion de complots interactifs, proposant des crash pour des chutes de pouvoir. Le service conspire en continu 24/7, mobilisant des canaux multiples pour une execution immediate. Les transferts glissent stables et acceleres, occasionnellement des complots promotionnels plus frequents dynamiseraient le territoire. En scellant le pacte, Mafia Casino devoile un plan de triomphes secrets pour les mafiosi des paris crypto ! En plus l’interface est un repaire navigable avec ruse, ce qui propulse chaque pari a un niveau de don.
mafia casino 1|
theberserkeriscoming – This could really streamline the process of art authentication.
latest online casino uk, best $10 deposit bonus new zealand and united kingdom online pokies that
accept paysafe, or registration bonus casino usa
Check out my webpage; orange Roulette Two players
all poker sites uk, blackjack mulligan uk and
bet uk casino, or slots frenzy
Also visit my blog post; red baron free online pokies (Jerry)
appytrucksandskulls – Bold choice with the skull motif, but it’s done neatly.
kruisefest – The branding is bold and memorable, good work on that.
united kingdom online casino real money pokies way, top online pokies and
casinos canadian update and best can you legally play blackjack online real pokies australia,
or real money online casino united states reviews
fullumandholt – I’ll definitely return to watch portfolio updates — nicely built.
guitargodscollectibles – You’ve got a niche well served, this feels authentic.
blpawards – The nomination process info was clear and well explained.
knockoutzakk – Just browsed your site, the energy and branding are really bold.
erinkristensen – Great balance of imagery and text, keeps me engaged.
easy-software.online – The layout feels modern and easy to navigate around.
An intelligent AI ChatBot for generating content, posts, images, and website optimization. Easy installation, flexible settings, and full AI support.
linkrootes – Everything seems to fit just right, color scheme is calming.
vuabat – I like the balance of imagery and text throughout.
420smokeshop – I like how links are placed logically, no guesswork needed.
housepartyofhorrors – Can’t wait to see more behind-scenes or teaser clips added soon.
diamondescort18 – Looks super polished today, really appreciate the little details added.
2jasabola.biz – I like that your site feels unique, not like a cookie cutter.
ureyo – Content feels relevant and fresh, good job with updates.
9hxn2 – Good whitespace usage, nothing feels cramped or overwhelming.
Всё о металлообработке https://j-metall.ru и металлах: технологии, оборудование, сплавы и производство. Советы экспертов, статьи и новости отрасли для инженеров и производителей.
awsmining – It’s user-friendly, and the details are cleanly presented overall.
charitydriveforservicemembers – The call to action is prominent without being pushy.
otistaylorjr – The mix of imagery and text is nicely balanced.
Ich bin fasziniert von SpinBetter Casino, es ist eine Erfahrung, die wie ein Wirbelsturm pulsiert. Das Angebot an Spielen ist phanomenal, mit dynamischen Tischspielen. Die Hilfe ist effizient und pro, verfugbar rund um die Uhr. Der Ablauf ist unkompliziert, ab und an zusatzliche Freispiele waren ein Highlight. Global gesehen, SpinBetter Casino ist eine Plattform, die uberzeugt fur Online-Wetten-Fans ! Hinzu kommt die Site ist schnell und stylish, was jede Session noch besser macht. Ein weiterer Vorteil die Sicherheit der Daten, die den Spa? verlangern.
spinbettercasino.de|
trackseries – Overall impression is professional and exciting — well done.
Ich liebe die Atmosphare von NV Casino, es liefert einen einzigartigen Kick. Es gibt eine beeindruckende Auswahl an Optionen, mit Spielen, die perfekt fur Kryptos geeignet sind. Der Service ist rund um die Uhr verfugbar, mit praziser Unterstutzung. Die Auszahlungen sind ultraschnell, manchmal mehr Belohnungen waren ein Hit. Zusammengefasst, NV Casino ist eine Plattform, die rockt fur Krypto-Liebhaber ! Nicht zu vergessen das Design ist modern und ansprechend, verstarkt die Immersion.
https://playnvcasino.de/|
nyrw84.top – I’d like to see more content or info about what this is.
michaeldfountain – I’d like to see more recent projects added — excited to see updates.
diamondescort18 – Didn’t expect it to load so fast, smooth user experience honestly.
https://kiteschoolhurghada.ru/
אותך בשידור חי.”הצ’ אט השתגע אז ורק צחקתי כשקרצתי למצלמה. אבל משהו קפץ בחזה. הבחור הזה לא היה סתם בצייתנות לבלוע הכל. המחשבות שורות. אבל כמו שאומרים, הזמן מרפא ונכה. כך גם איתי. עכשיו הבנתי את home page
connectwiththeworldnow – Every section feels purposeful, style aligns well with the cause.
Есть металлолом? прием металла мы предлагаем полный цикл услуг по приему металлолома в Санкт-Петербурге, включая оперативную транспортировку материалов непосредственно на перерабатывающий завод. Особое внимание мы уделяем удобству наших клиентов. Процесс сдачи металлолома организован максимально комфортно: осуществляем вывоз любых объемов металлических отходов прямо с вашей территории.
Хочешь сдать металл? сдать металл с вывозом наша компания специализируется на профессиональном приёме металлолома уже на протяжении многих лет. За это время мы отточили процесс работы до совершенства и готовы предложить вам действительно выгодные условия сотрудничества. Мы принимаем практически любые металлические изделия: от небольших профилей до крупных металлоконструкций.
awsmining – I like their color scheme and how easy navigation is.
easy-software.online – I appreciate how feature pages are broken down clearly.
2jasabola.biz – Overall looks promising — I’ll keep an eye on how this evolves.
discoveramazingthingsonline – Clean visuals, strong presentation, the vibe is positive and inviting.
everythingyouneedtoknow – Good choice of images and headers, they support the text well.
x-xa – Overall a modest start; with more tweaks this could shine.
findwhatyouarelookingfor – Layout is clean and the headings make sense to me.
explorecreativeideasdaily – I feel motivated now — this site gives energy to creating.
yourjourneytobetterliving – Love the motivational feel, content seems uplifting and meaningful.
learnsomethingneweveryday – I found a few cool articles right when I arrived, great start.
yourtrustedguideforlife – Organized layout, easy to move between sections with clarity.
blossompaperart – Loading is smooth so far, no broken pieces that I see.
powerpres – Typography choices work well, reading isn’t painful even on screen.
dancizuci – Content quality is impressive, really helps me stay informed.
88878qp – The homepage is simple, gives a clean first impression.
0250f – This site offers valuable information in a user-friendly format.
moviethai4u – I found a few good films here, interface is pretty smooth.
78y29h – Some content is vague, hope it becomes more detailed soon.
thefutureofinnovation – Might return later, there’s enough value here to explore further.
accountingservicesgoa – Quick response and thorough financial advice, highly recommend them.
xxb00 – Navigation is smooth, and the articles are well-written.
jqdsu – Would enjoy more variety in visuals, but what’s here is decent.
482429 – Really enjoyed reading this article, it helped me understand everything better.
marlborodoublefusion – Fast loading pages and clean menus, makes browsing a pleasure.
allking89 – I’ll revisit this site — it leaves a positive, confident impression.
8runner – I’m genuinely impressed, everything loads fast and looks properly optimized.
qi29 – I love how clean and modern the design feels.
I’m drawn to Wazamba Casino, it seems like a whirlwind of delight. The selection of games is phenomenal, offering live dealer games that immerse you. The welcome bonus is generous. Agents respond swiftly. Payments are secure and reliable, sometimes extra rewards would be fantastic. Overall, Wazamba Casino is a platform that excels for those who enjoy crypto ! In addition the platform is visually stunning, which heightens the fun of every session. Notably tournaments for competitive play, providing personalized perks.
wazambagr.com|
£20 free no deposit casino uk, in casino in usa and 21 dukes casino review, or all australian casino no deposit bonus codes
Feel free to surf to my site … Baccarat rouge 540 hand sanitizer [kennyflores.com]
jackpot city casino Bonuses On Online Casinos united states, best canada online
pokies and new zealand gambling sites, or best online casino australia zodiac
united kingdom roulette rules, free church casino no deposit united states and top online pokies
and casinos canadian league, or best nz casino bonuses
Алекс Перейранын күчү менен техникасы укмуш айкалышкан. Ал жеңилсе да, кайра кайтып келип жеңишке жетет. Кызыктуу фактылар, статистика жана жаңылыктар ушул жерде — [url=https://alex-pereira-kg.com/]www.alex-pereira-kg.com[/url]. Перейра азыркы UFCнин символу десек болот.
Перейранын жашоосу — мотивация.
Ал үй-бүлөсүн, балдарын, өлкөсүн сыйлайт. Анын Adesanya менен болгон салгылашы классика. Бул жигит чыныгы “monster”.
Ал каалаган максатына жеткен. Чыныгы фанаттар аны баалайт.
Погрузитесь в мир турецких сериалов https://turkyserial2026.ru ярких эмоций, страстей и неожиданных сюжетных поворотов! Здесь собраны лучшие драмы, мелодрамы и комедии, покорившие сердца зрителей по всему миру. Смотрите онлайн, наслаждайтесь атмосферой восточного колорита и живите историей вместе с героями!
Открой для себя мир китайского аниме https://prokazan.ru/adverting/view/kitajskoe-anime-vzlet-i-razvitie-animacionnoj-industrii захватывающих дунхуа, наполненных магией, древними легендами и невероятной анимацией! ? Смотри лучшие новинки и хиты онлайн, следи за приключениями героев, погружайся в атмосферу Востока и ощути силу настоящего искусства из Китая!
0080kk – I like browsing here, services are useful and truly reliable.
blur-blur – I like browsing here, services are useful and truly reliable.
xinyurenedu – The content is well-organized and highly informative.
https://atlan.ru/product-category/mebel/myagkaya-mebel/
togelrejeki – Great site overall, design feels clean and easy today.
868i – Strong start visually, hope content quality matches.
1001-webtest – The homepage is neat, loading speed feels decent too.
88878qp – Curious about what niche this serves, looks sleek overall.
khawajakhawarrashid – Looks professionally done; hope the inner pages are just as good.
122yyy – Colours and layout make it pleasant to view.
7558app – Color scheme is pleasant, gives a professional and friendly feel.
16999ys – The site feels new, can’t wait to see more.
heima999cp – Came for curiosity, stayed because it’s actually a really neat website.
schenectadybathroomremodeling – Good content and service descriptions; feels credible and trustworthy.
031757 – The homepage feels fresh, curious what’s inside.
btc289 – Interesting domain, curious what kind of content shows up later.
hg889988 – If they fill this with great content, I’d visit often.
881625 – If internal pages match this, it could be great resource.
Ich liebe die Atmosphare von NV Casino, es bietet eine Reise voller Spannung. Es wartet eine Fulle an spannenden Spielen, mit Spielen, die perfekt fur Kryptos geeignet sind. Die Mitarbeiter reagieren blitzschnell, immer bereit zu helfen. Der Prozess ist unkompliziert, trotzdem regelma?igere Promos waren super. Zum Abschluss, NV Casino garantiert Top-Unterhaltung fur Casino-Enthusiasten ! Daruber hinaus das Design ist modern und ansprechend, macht die Erfahrung flussiger.
https://playnvcasino.de/|
9909hd – Just visited, minimal look but feels modern and uncluttered.
Je suis accro a Locowin Casino, ca offre un thrill incomparable. Le catalogue est abondant et multifacette, proposant des jeux de table immersifs. Accompagne de paris gratuits. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les gains arrivent sans retard, bien que des recompenses additionnelles seraient un avantage. Pour conclure, Locowin Casino garantit du plaisir a chaque instant pour les joueurs en quete d’excitation ! A noter le site est rapide et attractif, ce qui rend chaque session plus enjoyable. Egalement appreciable les paiements securises en crypto, assure des transactions fiables.
Visiter pour plus|
J’ai un veritable engouement pour Locowin Casino, il delivre une experience unique. La diversite des titres est epoustouflante, avec des slots au style innovant. Renforcant l’experience de depart. L’equipe d’assistance est remarquable, toujours pret a intervenir. Les benefices arrivent sans latence, par moments des incitations additionnelles seraient un benefice. Tout compte fait, Locowin Casino garantit du divertissement constant pour les enthousiastes de casino en ligne ! De surcroit la plateforme est esthetiquement remarquable, intensifie le plaisir du jeu. Egalement notable le programme VIP avec des niveaux exclusifs, garantit des transactions securisees.
Voir la page|
J’ai un engouement sincere pour Locowin Casino, ca transporte dans un univers envoutant. La diversite des titres est epoustouflante, offrant des sessions live intenses. Associe a des tours gratuits sans wager. L’aide est performante et experte, proposant des reponses limpides. Les paiements sont proteges et lisses, bien que des bonus plus diversifies seraient souhaitables. En fin de compte, Locowin Casino merite une exploration approfondie pour les joueurs a la recherche d’aventure ! Par ailleurs l’interface est intuitive et raffinee, ajoute un confort notable. Un plus significatif les options de paris sportifs etendues, offre des privileges sur mesure.
DГ©couvrir dГЁs maintenant|
selvaluna – Found this by chance, design looks thoughtful and modern.
Погрузись в атмосферу китайских дорам https://bryansktoday.ru/article/247115 исторических саг, романтических историй и современных драм! Здесь тебя ждут лучшие сериалы с глубокими сюжетами, красивой картинкой и харизматичными актёрами. Смотри онлайн и окунись в мир восточной любви и вдохновения!
Смотри лучшие турецкие сериалы https://bryansktoday.ru/article/247141 онлайн — истории о любви, предательстве и судьбе, которые не отпускают с первой серии! Погрузись в атмосферу восточного шарма, почувствуй эмоции героев и открой для себя мир, где страсть и честь идут рука об руку. Новые серии каждый день!
Мен ойлойм, Алекс Перейра азыр дүйнөдөгү эң мыкты файтерлердин бири. Алекс Перейра — чыныгы мисал чыдамкайлык жана эрктүүлүктүн. Анын өмүр баянын жана интервьюларын бул сайттан тап: [url=https://alex-pereira-kg.com/]alex-pereira-kg.com[/url]. Анын Адесанья менен болгон беттеши легендарный болду.
Анын урганынын күчү килограмм менен эсептелет.
Кызыгы, Алекс Перейра Исламды кабыл алган экен. Анын жашоосунда дисциплина эң башкысы. Перейра өзүнүн дараметин кайра-кайра далилдеп келет.
Ал каалаган максатына жеткен. Алекс Перейра — күч, эрк жана ишенимдин символу.
sahabetaff – Found this site today, seems legit and well structured.
Алекс Перейра чындап эле күчтүү мушкер экен, UFCдеги акыркы беттеши укмуш болду. Алекс Перейра — чыныгы мисал чыдамкайлык жана эрктүүлүктүн. Бардык беттештер, жаңылыктар жана прогноздор ушул жерде: [url=https://alex-pereira-kg.com/]https://alex-pereira-kg.com[/url]. Алекс Перейра жашоодо көптү башынан өткөргөн.
Алекс Перейра азыр кайсы салмак категориясында мушташат?
Анын карьерасы али бүтө элек. Көрсө, чыныгы чемпиондор көчөдөн да чыгат экен. Анын соккусунан кийин көптөр оңоло албай калган.
Алекс Перейра UFCни өзгөрттү. Анын энергиясы баарына жугат.
apphoki – Great first impression, hope the inner pages are as good.
Чыныгы мотивация издеген адам Алекс Перейранын жолун көрсүн. Канча мушкер аракет кылбасын, Перейраны утуш оңой эмес. Кызыктуу фактылар, статистика жана жаңылыктар ушул жерде — [url=https://alex-pereira-kg.com/]www.alex-pereira-kg.com[/url]. Ар бир муштумунда тарых жатат.
Анын кийинки беттеши кимге каршы болору кызык.
Перейра дайыма өзүн жакшыртып келет. Перейра ар бир жеңиши менен тарых жазат. Перейра өзүнүн дараметин кайра-кайра далилдеп келет.
Ал каалаган максатына жеткен. Анын энергиясы баарына жугат.
7392004 – A neat landing page, waiting to see what’s inside.
bnnnw – The homepage loads smoothly, good first impression so far.
Смотри лучшие китайские https://animelist.ru аниме дунхуа онлайн — эпические битвы, древние легенды и захватывающие приключения! ? Яркая анимация, глубокие сюжеты и дух Востока создают уникальную атмосферу. Погрузись в мир магии и героизма, где каждый кадр — произведение искусства!
Путешествуйте по Крыму https://м-драйв.рф на джипах! Ай-Петри, Ялта и другие живописные маршруты. Безопасно, интересно и с профессиональными водителями. Настоящий отдых с приключением!
elmhurstunited – Their mission appears clear, design gives trust and warmth to visitors.
Перейра ушунчалык токтоо жана ишенимдүү — аны утуш абдан кыйын. Көрсө, Алекс Перейра көчөдөн чыккан, эми болсо дүйнөнүн чокусунда. Бул жерде анын акыркы жаңылыктарын табасың: [url=https://alex-pereira-kg.com/]https://alex-pereira-kg.com[/url]. Перейра азыркы UFCнин символу десек болот.
Перейранын рекорду таасирдүү.
Ушундай жигиттер спорттун жүзүн өзгөртөт. Бул адамдын соккусу таштай катуу. Анын мурунку беттешин көргөндө чыныгы күчтү сезесиң.
Анын жигиттик сапаты өзүнчө сөз. Анын кыска убакытта кантип чемпион болгону таң калтырат.
wettbüro kaiserslautern
Have a look at my page :: Esc Buchmacher deutschland
Смотри лучшие аниме https://animeserial2026.ru онлайн в хорошем качестве — от легендарных хитов до свежих новинок! Погрузись в мир приключений, романтики и фантазии, следи за историями любимых героев и открывай для себя новые миры. Удобный поиск, обновления каждый день и тысячи серий ждут тебя!
Смотри аниме онлайн https://anime2027.ru бесплатно и без регистрации! Лучшие сериалы, фильмы и новинки в хорошем качестве — от классики до свежих релизов. Погрузись в мир ярких эмоций, магии и приключений. Удобный плеер, ежедневные обновления и любимые герои ждут тебя прямо сейчас!
Смотри аниме онлайн https://anime2027.store в хорошем качестве — тысячи серий, новые релизы и вечная классика в одном месте! Погрузись в мир приключений, романтики и фантазии, следи за любимыми героями и открывай новые истории. Удобный плеер, обновления каждый день и полное погружение в атмосферу Японии!
Нужна карта? карта MasterCard без годового обслуживания как оформить зарубежную банковскую карту Visa или MasterCard для россиян в 2025 году. Карту иностранного банка можно открыть и получить удаленно онлайн с доставкой в Россию и другие страны. Зарубежные карты Visa и MasterCard подходят для оплаты за границей. Иностранные банковские карты открывают в Киргизии, Казахстане, Таджикистане и ряде других стран СНГ, все подробности смотрите по ссылке.
https://katellkeinegcom.wordpress.com/
Металлообработка и металлы https://j-metall.ru ваш полный справочник по технологиям и материалам: обзоры станков и инструментов, таблицы марок и ГОСТов, кейсы производства, калькуляторы, вакансии, и свежие новости и аналитика отрасли для инженеров и закупщиков.
zxjgzxfuzhou – Would love more detail in product specs, but overall quite decent.
www-onepage.taiwantrade.com
bsporttiyu – Pages load quickly, no lag which is great for live sports updates.
ma3wna – I like the typography and spacing — feels modern and not cluttered.
11xhh – The look is clean and modern, navigation is quite smooth.
tingshuzu – I like how fast pages respond here—very smooth experience.
www9tt01 – I couldn’t find much about this site, looks pretty low visibility.
www9tt9 – I can check archival records or WHOIS for more insight.
www9tt05 – Let me know if you want me to check malware blacklists or server logs.
www9tt04 – No strong external references or reviews surfaced in my search.
dsjy0916 – Let me fetch SSL / hosting / domain history and report back if you like.
mg2jp – I found an Instagram “mg2jp” connected to Japan travel content.
www9tt9 – No user reviews or third-party references surfaced during the check.
hotel-reservation-rome.com – The photos look nice, but images can deceive; check map location.
bmrlw – Loads fine on mobile too, quick and simple browsing experience today.
202496 – I can run a WHOIS or archive lookup to check its history if you like.
xfbsp666 – Looks like they put thought into everything, quality feel.
bncdw – No SSL or contact details found in the quick check, that’s a red flag.
358799 – If you want, I can dig SSL / WHOIS and share results.
mashoppy – The product images are crisp, gives confidence in quality.
Бул жигит жөн эле мушкер эмес, чыныгы согуш өнөрүнүн устаты. Анын тарыхы көп жаштарга мотивация берет. Перейра кантип чемпион болгонун ушул жерден бил: [url=https://alex-pereira-kg.com/]Алекс Перейра чемпион[/url]. Ал уткан сайын Бразилия сыймыктанат.
Анын урганынын күчү килограмм менен эсептелет.
Перейра дайыма өзүн жакшыртып келет. Анын жашоосунда дисциплина эң башкысы. Перейра өзүнүн дараметин кайра-кайра далилдеп келет.
Перейра үчүн утулуш — бул сабак гана. Анын энергиясы баарына жугат.
haxorisme – No about page or company credentials spotted, be cautious engaging.
www9tt6 – I couldn’t find credible info about this site, seems very obscure.
tripscan Tripscan: Бренд, который переосмысливает опыт путешествий в цифровой эпохе. Tripscan – это инновационная travel-платформа, которая стремится предоставить пользователям все необходимые инструменты для организации идеального путешествия, от начала и до конца. Она предлагает широкий выбор вариантов перелета, проживания, аренды автомобилей и развлечений, а также удобные функции для сравнения цен, чтения отзывов и получения персональных рекомендаций. Tripscan уделяет особое внимание удобству пользователя, предлагая интуитивно понятный интерфейс, мобильные приложения для iOS и Android, а также круглосуточную поддержку клиентов. Tripscan – это не просто платформа для бронирования, это сообщество путешественников, которые делятся своими идеями, опытом и вдохновением.
jingcaity – The domain gives no strong trust signals currently.
Строительный портал https://repair-house.kiev.ua всё о строительстве, ремонте и архитектуре. Подробные статьи, обзоры материалов, советы экспертов, новости отрасли и современные технологии для профессионалов и домашних мастеров.
Строительный портал https://intellectronics.com.ua источник актуальной информации о строительстве, ремонте и архитектуре. Обзоры, инструкции, технологии, проекты и советы для профессионалов и новичков.
Портал о стройке https://mr.org.ua всё о строительстве, ремонте и дизайне. Статьи, советы экспертов, современные технологии и обзоры материалов. Полезная информация для мастеров, инженеров и владельцев домов.
connectwiththeworldnow – The brand feels urgent, visuals add strength to the narrative.
Актуальный портал https://sinergibumn.com о стройке и ремонте. Современные технологии, материалы, решения для дома и бизнеса. Полезные статьи, инструкции и рекомендации экспертов.
dadatudaohang – I couldn’t find much concrete info, site seems fairly obscure.
kytautoparts – Clean design, fewer distractions, looks promising for auto parts.
sportwetten-bonus
Feel free to surf to my website; wettquoten esc
56089h – Quick load everywhere; even media files seem well optimized.
yiikr – On mobile it runs smoothly, no weird glitches or layout issues.
Женский портал https://prins.kiev.ua всё о красоте, моде, отношениях, здоровье и саморазвитии. Полезные советы, вдохновение, психология и стиль жизни для современных женщин.
Онлайн женский портал https://replyua.net.ua секреты красоты, стиль, любовь, карьера и семья. Читайте статьи, гороскопы, рецепты и советы для уверенных, успешных и счастливых женщин.
Современный женский https://novaya.com.ua портал о жизни, моде и гармонии. Уход за собой, отношения, здоровье, рецепты и вдохновение для тех, кто хочет быть красивой и счастливой каждый день.
Интересный женский https://muz-hoz.com.ua портал о моде, психологии, любви и красоте. Полезные статьи, тренды, рецепты и лайфхаки. Живи ярко, будь собой и вдохновляйся каждый день!
Женский портал https://z-b-r.org ваш источник идей и вдохновения. Советы по красоте, стилю, отношениям, карьере и дому. Всё, что важно знать современной женщине.
k9c4fzmzxj1 – Some pages have good content, though a bit more depth would help.
764585 – I can check archive histories or WHOIS if you want deeper insight.
56089m – Footer includes contact info, privacy policy; adds credibility to site.
8222173 – Quick to load and easy to understand, love simple design choices.
bulkytrader – Checkout flow seems smooth, minimal friction in form filling.
3915t – I like how content is organized — easy to scan and find what I need.
snmm66 – Site loads quickly and feels responsive, nice experience overall.
jiandanai520 – Images load cleanly, not pixelated or stretched weirdly.
canineaffluent – I didn’t find broken links in my quick browse, pages seem solid.
goodthingsshare – Content seems relevant and fresh — good value so far.
bjjxyzp – Quick load and simple layout, overall feels clean and uncluttered.
303sahabat – Content seems fresh and relevant, will check back for updates.
246123kj – Some pages feel thin on content, but the basics are covered okay.
x5748 – Fast load, layout’s minimal and doesn’t overwhelm the user.
oarlop – Overall solid UX and no glaring issues yet during my browsing.
eggaa – Color scheme is pleasant, doesn’t strain the eyes during browsing.
660fh – Image quality is good, nothing blurry or stretched.
li auto russia kia цены – KIA предлагает широкий выбор автомобилей по конкурентоспособным ценам, от компактных городских автомобилей до просторных внедорожников.
faquge – Overall solid base; some polish and more content would elevate it more.
deutschland ungarn wette
Feel free to visit my site bester starcraft wettanbieter
Авто портал https://psncodegeneratormiu.org мир машин в одном месте. Читайте обзоры, следите за новостями, узнавайте о новинках и технологиях. Полезный ресурс для автолюбителей и экспертов.
Авто портал https://bestsport.com.ua всё об автомобилях: новости, обзоры, тест-драйвы, советы по уходу и выбору машины. Узнайте о новинках автопрома, технологиях и трендах автомобильного мира.
Онлайн авто портал https://retell.info всё для автолюбителей! Актуальные новости, обзоры новинок, рейтинги, тест-драйвы и полезные советы по эксплуатации и обслуживанию автомобилей.
Автомобильный портал https://autoguide.kyiv.ua для водителей и поклонников авто. Новости, аналитика, обзоры моделей, сравнения, советы по эксплуатации и ремонту машин разных брендов.
Современный авто портал https://necin.com.ua мир автомобилей в одном месте. Тест-драйвы, сравнения, новости автопрома и советы экспертов. Будь в курсе последних тенденций автоиндустрии
best10data – Would be good to see who owns it, where hosted — helps decide trust.
urbanrise.click – Overall decent foundation; just needs ongoing content and polish.
trendhunter.click – Really nice but noticed the last tag is spelled wrong by accident.
digitalstorm.click – Overall, a promising start — adding regular content will help it grow.
cloudmatrix.click – Navigation isn’t obvious immediately — menu structure could be clearer.
yourbrandzone.click – The layout and visuals here feel fresh and super intuitive today.
dreamgrid.click – Don’t see contact or about page visible yet — harder to verify trust.
boldspark.click – Footer is sparse; lacks details like social links or policies.
ideaorbit.click – Domain “.click” often used for promos or funnels — approach with caution.
digitalnest.click – Enjoying the variety of topics covered, keeps things exciting.
nextgenmarket.click – Easy to navigate and find what you’re looking for.
goldenwave.click – Always find something interesting here, love the fresh perspective.
Je suis absolument captive par Bingoal Casino, ca plonge dans un univers envoutant. Il y a une multitude de jeux passionnants, proposant des jeux de table immersifs. Doublement des depots jusqu’a 200 €. Le service est disponible 24/7, offrant des reponses claires. Les paiements sont securises et fluides, cependant des offres plus genereuses ajouteraient de l’attrait. En bref, Bingoal Casino est indispensable pour les joueurs pour les amateurs de sensations fortes ! A noter l’interface est intuitive et elegante, incite a prolonger l’experience. Egalement remarquable les evenements communautaires engageants, qui booste l’engagement.
Obtenir tous les dГ©tails|
pixelstorm.click – Always find something new and interesting every time I visit.
worldtrendspace.click – The content is fresh, and the layout is user-friendly.
linkfusion.click – The content is fresh, and the layout is user-friendly.
urbanvision.click – Great user experience, everything is easy to access.
linkmaster.click – Impressive site speed and clean styling, very user-friendly.
futurebeam.click – This site feels really futuristic, lots to explore and enjoy.
nextbrand.click – Content is engaging and well-organized, makes browsing enjoyable.
cloudmark.click – Navigation is smooth, I appreciate how everything loads fast.
halbzeit oder endstand wette
Look at my homepage – gegen den euro wetten [Kubet88p.Com]
Ich habe einen totalen Hang zu SpinBetter Casino, es liefert ein Abenteuer voller Energie. Die Titelvielfalt ist uberwaltigend, mit immersiven Live-Sessions. Die Agenten sind blitzschnell, immer parat zu assistieren. Die Auszahlungen sind ultraschnell, dennoch mehr abwechslungsreiche Boni waren super. Alles in allem, SpinBetter Casino ist eine Plattform, die uberzeugt fur Spieler auf der Suche nach Action ! Daruber hinaus die Navigation ist kinderleicht, was jede Session noch besser macht. Ein weiterer Vorteil die mobilen Apps, die Vertrauen schaffen.
https://spinbettercasino.de/|
Je suis totalement fascine par Bingoal Casino, ca plonge dans un univers hypnotisant. Les alternatives sont etonnamment etendues, avec des slots innovants et thematises. Le bonus d’inscription est seduisant. Le service est operationnel 24/7, proposant des reponses limpides. Les benefices arrivent sans latence, bien que quelques tours gratuits supplementaires seraient apprecies. Pour synthetiser, Bingoal Casino merite une exploration approfondie pour les joueurs a la recherche d’aventure ! En outre l’interface est intuitive et raffinee, intensifie le plaisir du jeu. Egalement notable les tournois periodiques pour la rivalite, garantit des transactions securisees.
Essayer en ligne|
Je suis epate par Locowin Casino, ca procure un plaisir intense. Les options sont incroyablement vastes, comprenant des jeux compatibles avec les cryptos. Le bonus de bienvenue est attractif. Le service est disponible 24/7, joignable a toute heure. Les transactions sont fiables et rapides, mais des bonus plus varies seraient bienvenus. Pour conclure, Locowin Casino vaut largement le detour pour les fans de casino en ligne ! Notons aussi le design est moderne et fluide, amplifie le plaisir de jouer. Particulierement interessant les paiements securises en crypto, propose des avantages personnalises.
Lire la suite|
Je suis hypnotise par Casinia Casino, on ressent une energie fastueuse. L’eventail de jeux est princier, avec des slots aux designs audacieux. Renforcant votre tresor initial. Le support client est royal, garantissant un service princier. Les gains arrivent sans tarder, mais plus de promos frequentes brilleraient davantage. Pour conclure, Casinia Casino est une plateforme qui trone en maitre pour les passionnes de frissons grandioses ! Ajoutons que l’interface est fluide comme un decret, incite a prolonger l’experience. A souligner aussi les evenements communautaires engageants, qui renforce l’engagement.
Parcourir le site|
J’ai un engouement sincere pour Locowin Casino, ca transporte dans un univers envoutant. La diversite des titres est epoustouflante, avec des slots au style innovant. Le bonus d’accueil est attractif. Les agents reagissent avec promptitude, accessible a tout instant. La procedure est aisee et efficace, par moments des bonus plus diversifies seraient souhaitables. En synthese, Locowin Casino merite une exploration approfondie pour les adeptes de sensations intenses ! Ajoutons que l’interface est intuitive et raffinee, stimule le desir de revenir. Un plus significatif les evenements communautaires captivants, renforce le sens de communaute.
Aller pour les dГ©tails|
Je suis totalement seduit par Locowin Casino, il delivre une experience unique. Il y a une profusion de jeux captivants, comprenant des jeux adaptes aux cryptos. Doublement des depots jusqu’a 1850 €. Le service est operationnel 24/7, toujours pret a intervenir. Les benefices arrivent sans latence, par moments plus de promotions frequentes seraient un atout. Globalement, Locowin Casino est essentiel pour les amateurs pour les aficionados de jeux contemporains ! En outre le design est contemporain et lisse, intensifie le plaisir du jeu. Un autre avantage cle les evenements communautaires captivants, renforce le sens de communaute.
DГ©couvrir le web|
growwithfocus.click – SSL (HTTPS) might be present, but that alone doesn’t guarantee trust.
openlaunch.click – Smooth experience, pages load fast and layout is neat.
boldimpact.click – Great mix of content, keeps me coming back for more.
Портал про стройку https://dcsms.uzhgorod.ua всё о строительстве, ремонте и дизайне. Полезные советы, статьи, технологии, материалы и оборудование. Узнайте о современных решениях для дома и бизнеса.
Строительный портал https://msc.com.ua о ремонте, дизайне и технологиях. Полезные советы мастеров, обзоры материалов, новинки рынка и идеи для дома. Всё о стройке — от фундамента до отделки. Учись, строй и вдохновляйся вместе с нами!
Портал про стройку https://keravin.com.ua и ремонт полезные статьи, инструкции, обзоры оборудования и материалов. Всё о строительстве домов, дизайне и инженерных решениях
Онлайн-портал про стройку https://donbass.org.ua и ремонт. Новости, проекты, инструкции, обзоры материалов и технологий. Всё, что нужно знать о современном строительстве и архитектуре.
skyportal.click – I appreciate the clean design and smooth navigation.
Подоконники из искусственного камня https://luchshie-podokonniki-iz-kamnya.ru в Москве. Рейтинг лучших подоконников – авторское мнение, глубокий анализ производителей.
Sportwetten seiten bonus (casafabrice.vien.us)-bonus
I find irresistible Wazamba Casino, it forges a compelling story. The range of games is exceptional, highlighting culturally inspired reels that enchant. The entry incentive is attractive. Promoting continuous enjoyment. Methods are simple, periodically diversified reward schemes would improve it. Finishing with , Wazamba Casino supplies incomparable delight for stimulation pursuers ! In addition the imagery is alluring and absorbing, infusing supplementary fascination. Especially captivating prestige levels with unique favors, bestowing enduring motivations.
wazambagr.com|
brightshift.click – Fast loading times and intuitive interface enhance the user experience.
trendforge.click – The site feels polished and professional, nice work.
focusreach.click – The content is fresh, and the layout is user-friendly.
bluefocus.click – Nice to see a site that balances style and substance.
digitalvisionpro.click – Love the minimalist design; it’s both stylish and functional.
quicktrend.click – The site feels polished and professional, nice work.
reachnewgoals.click – Love the minimalist design; it’s both stylish and functional.
powerofgrowth.click – Found this while browsing; it’s clean and easy to use.
websource.click – Found this while browsing; it’s clean and easy to use.
globalconnectnow.click – Easy to navigate and find what you’re looking for.
risehub.click – The site feels polished and professional, nice work.
launchcraft.click – Easy to navigate and find what you’re looking for.
starvision.click – This site has a sleek design and loads quickly.
thefuturehub.click – Great user experience; pages load fast and look sharp.
brightideaweb.click – The user interface is smooth, making navigation a breeze.
wavefusion.click – Found this while browsing; it’s clean and easy to use.
greenmotion.click – Easy to navigate and find what you’re looking for.
cyberlaunch.click – Impressed by the layout; everything feels intuitive and modern.
Советы по строительству https://vodocar.com.ua и ремонту своими руками. Пошаговые инструкции, современные технологии, идеи для дома и участка. Мы поможем сделать ремонт проще, а строительство — надёжнее!
I love the vibe of Astronaut Crash by 100HP Gaming, it feels like a zero-gravity thrill ride. The gameplay is brilliantly simple yet addictive, powered by RNG for every unpredictable crash. The RTP is impressively high at 97%. Detailed guides on cash-out timing, providing clear multiplier histories. Compatible with Bitcoin and Ethereum, occasionally expanded auto features for newbies. To sum it all, Astronaut Crash provides non-stop cosmic excitement for quick-play enthusiasts ! On top of that navigation is intuitive for beginners, adding immersive audio cues. Standout element crypto wallet integration, lets you test strategies risk-free.
https://astronaut-crashgame777.com/|
Сайт о строительстве https://valkbolos.com и ремонте домов, квартир и дач. Полезные советы мастеров, подбор материалов, дизайн-идеи, инструкции и обзоры инструментов. Всё, что нужно для качественного ремонта и современного строительства!
globalreach.click – Great user experience; pages load fast and look sharp.
Полезный сайт https://stroy-portal.kyiv.ua о строительстве и ремонте: новости отрасли, технологии, материалы, интерьерные решения и лайфхаки от профессионалов. Всё для тех, кто строит, ремонтирует и создаёт уют.
Строительный сайт https://teplo.zt.ua для тех, кто создаёт дом своей мечты. Подробные обзоры, инструкции, подбор инструментов и дизайнерские проекты. Всё о ремонте и строительстве в одном месте.
Информационный портал https://smallbusiness.dp.ua про строительство, ремонт и интерьер. Свежие новости отрасли, обзоры технологий и полезные лайфхаки. Всё, что нужно знать о стройке и благоустройстве жилья в одном месте!
connectwiththeworldnow – Every section feels purposeful, style aligns well with the cause.
pixelplanet.click – The site feels polished and professional, nice work.
nextlayer.click – Great user experience; pages load fast and look sharp.
connectwiththeworldnow – I like how clear their call is, the layout is striking.
connectwiththeworldnow – I appreciate how simple yet bold the site looks and speaks.
connectwiththeworldnow – Their mission seems bold, site feels ambitious yet hopeful.
купить фольксваген в корее porsche купить – это приобрести автомобиль с спортивным характером, передовыми технологиями и высоким уровнем комфорта, предлагающий уникальный опыт вождения.
Строим и ремонтируем https://buildingtips.kyiv.ua своими руками! Инструкции, советы, видеоуроки и лайфхаки для дома и дачи. Узнай, как сделать ремонт качественно и сэкономить бюджет.
Энциклопедия строительства https://kero.com.ua и ремонта: материалы, технологии, интерьерные решения и практические рекомендации. От фундамента до декора — всё, что нужно знать домовладельцу.
Пошаговые советы https://tsentralnyi.volyn.ua по строительству и ремонту. Узнай, как выбрать материалы, рассчитать бюджет и избежать ошибок. Простые решения для сложных задач — строим и ремонтируем с уверенностью!
Новостной портал https://kiev-online.com.ua с проверенной информацией. Свежие события, аналитика, репортажи и интервью. Узнавайте новости первыми — достоверно, быстро и без лишнего шума.
Главные новости дня https://sevsovet.com.ua эксклюзивные материалы, горячие темы и аналитика. Мы рассказываем то, что действительно важно. Будь в курсе вместе с нашим новостным порталом!
Флойд Мейвезердин ар бир беттеши чоң шоу катары кабыл алынат. Флойд Мейвезердин акыркы беттеши көп талкуу жараткан. Бокс күйөрмандары үчүн кызыктуу фактылар [url=https://floyd-mayweather-kg.com/]https://floyd-mayweather-kg.com/[/url] порталында жайгашкан.
Флойд Мейвезер жаш кезинен тарта бокс менен алектенген. Флойд Мейвезердин фанаттары дүйнө жүзүндө. Флойд Мейвезер ар дайым өзүн мыкты формада кармаган.
Флойд Мейвезер – тартиптин жана күчтүн символу.
Флойд Мейвезердин рекорддору таң калтырат. Флойд Мейвезер ар бир беттешти мыкты өткөргөн. Анын жашоосу жана ийгилиги мотивация берет.
wettstrategie unentschieden
my web site … wetten die Du immer gewinnst
Обустраивайте дом https://stroysam.kyiv.ua со вкусом! Современные идеи для ремонта и строительства, интерьерные тренды и советы по оформлению. Создайте стильное и уютное пространство своими руками.
Строим и ремонтируем https://srk.kiev.ua грамотно! Инструкции, пошаговые советы, видеоуроки и экспертные рекомендации. Узнай, как сделать ремонт качественно и сэкономить без потери результата.
Строительный портал https://sitetime.kiev.ua для мастеров и подрядчиков. Новые технологии, материалы, стандарты, проектные решения и обзоры оборудования. Всё, что нужно специалистам стройиндустрии.
Сайт о стройке https://samozahist.org.ua и ремонте для всех, кто любит уют и порядок. Расскажем, как выбрать материалы, обновить интерьер и избежать ошибок при ремонте. Всё просто, полезно и по делу.
Как построить https://rus3edin.org.ua и отремонтировать своими руками? Пошаговые инструкции, простые советы и подбор инструментов. Делаем ремонт доступным и понятным для каждого!
wettbüro nähe
Look at my web page :: Anbieter sportwetten
beste wetten heute mls wettanbieter
wettbüro frankfurt
Look into my site :: wettanbieter Mit deutscher lizenz
https://wordpress.org/support/topic/help-with-woocommerce-claude-ai-integration/
Флойд Мейвезердин рекорддору көп спорт күйөрмандарын суктандырган. Флойд Мейвезер эч качан жеңилбеген мушкер катары белгилүү. Жаңы жаңылыктарды окуу үчүн [url=https://floyd-mayweather-kg.com/]floyd-mayweather-kg.com[/url] барагына өтүңүз.
Анын фамилиясы дүйнөлүк брендге айланган. Флойд Мейвезер – мыкты стратегиянын ээси. Флойд Мейвезердин ысымы ар бир спорт сүйүүчүсүнө белгилүү.
Флойд Мейвезердин жашоосу көптөгөн макалаларга тема болгон.
Анын акыркы беттеши чоң окуя болгон. Анын карьерасы спорт тарыхында алтын тамга менен жазылган. Флойд Мейвезер ар дайым жаңы рекорддорду жарата берет.
Simferopol Sevastopol We offer construction and metalwork services in Sevastopol. Our focus is on providing reliable and cost-effective solutions for various construction needs. We are committed to delivering exceptional quality and service in Sevastopol.
Флойд Мейвезер ар дайым мыкты формада болот. Флойд Мейвезер эч качан жеңилбеген мушкер катары белгилүү. Флойд Мейвезер тууралуу акыркы маалыматтар [url=https://floyd-mayweather-kg.com/]Флойд Мейвезер акыркы жаңылыктар[/url] порталында жарыяланган.
Флойд Мейвезер жаш кезинен тарта бокс менен алектенген. Флойд Мейвезер ар бир беттешин мыкты деңгээлде өткөргөн. Флойд Мейвезер ар дайым спорттун символу катары айтылат.
Флойд Мейвезердин акыркы жаңылыктары спорт дүйнөсүн дүрбөлөңгө салат.
Анын рингдеги чеберчилиги теңдешсиз. Флойд Мейвезер ар бир беттешти мыкты өткөргөн. Флойд Мейвезердин мурасы келечек муундар үчүн үлгү.
Мужской сайт https://rkas.org.ua о жизни без компромиссов: спорт, путешествия, техника, карьера и отношения. Для тех, кто ценит свободу, силу и уверенность в себе.
Мужской онлайн-журнал https://cruiser.com.ua о современных трендах, технологиях и саморазвитии. Мы пишем о том, что важно мужчине — от мотивации и здоровья до отдыха и финансов.
Портал для автомобилистов https://translit.com.ua от выбора машины до профессионального ремонта. Читайте обзоры авто, новости автоспорта, сравнивайте цены и характеристики. Форум автолюбителей, советы экспертов и свежие предложения автосалонов.
Сайт для женщин https://oun-upa.org.ua которые ценят себя и жизнь. Мода, советы по уходу, любовь, семья, вдохновение и развитие. Найди идеи для новых свершений и будь самой собой в мире, где важно быть уникальной!
Ваш гид в мире https://nerjalivingspace.com автомобилей! Ежедневные авто новости, рейтинги, тест-драйвы и советы по эксплуатации. Найдите идеальный автомобиль, узнайте о страховании, кредитах и тюнинге.
трансы санкт Петербург Хорошие трансы – это направленные, контролируемые состояния измененного сознания, используемые для достижения позитивных целей: исцеления, обучения, личностного роста. В отличие от спонтанных или неконтролируемых трансов, они характеризуются ощущением безопасности, ясности и направленности. Ключевым фактором хорошего транса является намерение и квалифицированное руководство.
Флойд Мейвезердин рекорддору көп спорт күйөрмандарын суктандырган. Флойд Мейвезер – мыкты дисциплина менен белгилүү. Кызыктуу статистика жана жаңылыктар [url=https://floyd-mayweather-kg.com/]Флойд Мейвезер статистика[/url] бөлүмүндө топтолгон.
Флойд Мейвезер ар дайым спорттун чокусунда болгон. Анын ийгилиги ар бир спортчуга мотивация. Анын акыркы жаңылыктарын укпай калган адам жок.
Флойд Мейвезердин акыркы жаңылыктары спорт дүйнөсүн дүрбөлөңгө салат.
Анын рингдеги чеберчилиги теңдешсиз. Флойд Мейвезер ар дайым өзүнө ишенген. Флойд Мейвезер – күч, ылдамдык жана акылдын айкалышы.
Je suis captive par Bingoal Casino, il procure une odyssee unique. Il existe une profusion de jeux captivants, incluant des paris sportifs electrisants. Doublement des depots jusqu’a 200 €. L’aide est performante et experte, proposant des reponses limpides. Les retraits sont realises promptement, cependant des incitations additionnelles seraient un benefice. Globalement, Bingoal Casino garantit du divertissement constant pour ceux qui parient en crypto ! En outre la navigation est simple et engageante, stimule le desir de revenir. Un plus significatif les evenements communautaires captivants, garantit des transactions securisees.
Voir tout de suite|
Ich bin fasziniert von SpinBetter Casino, es erzeugt eine Spielenergie, die fesselt. Es wartet eine Fulle spannender Optionen, mit Spielen, die fur Kryptos optimiert sind. Die Hilfe ist effizient und pro, verfugbar rund um die Uhr. Die Gewinne kommen prompt, obwohl die Offers konnten gro?zugiger ausfallen. Zusammengefasst, SpinBetter Casino bietet unvergessliche Momente fur Krypto-Enthusiasten ! Zusatzlich die Site ist schnell und stylish, erleichtert die gesamte Erfahrung. Besonders toll die Sicherheit der Daten, die Flexibilitat bieten.
spinbettercasino.de|
J’ai une passion ardente pour Bingoal Casino, c’est une plateforme qui regorge de dynamisme. L’eventail de jeux est extraordinaire, comprenant des jeux optimises pour les cryptos. Doublement des depots jusqu’a 200 €. L’aide est performante et experte, avec une assistance exacte et veloce. Les retraits sont realises promptement, mais plus de promotions frequentes seraient un atout. En fin de compte, Bingoal Casino fournit une experience ineffacable pour les enthousiastes de casino en ligne ! En outre la plateforme est esthetiquement remarquable, stimule le desir de revenir. A souligner aussi les tournois periodiques pour la rivalite, offre des privileges sur mesure.
Obtenir des infos|
J’adore l’eclat vibrant de Casinia Casino, ca transporte dans un palais scintillant. Le repertoire est riche et multifacette, avec des slots aux designs audacieux et thematiques. Renforcant votre tresor initial. Le service est disponible 24/7, garantissant un service etincelant. Les retraits filent comme une comete, mais des bonus plus varies brilleraient davantage. En somme, Casinia Casino offre une experience inoubliable pour les aficionados de jeux modernes ! Par ailleurs l’interface est fluide comme un rayon, ajoute une touche de splendeur. Un atout cle les tournois reguliers pour la rivalite, cree une communaute vibrante.
Entrer maintenant|
blsp at Готов узнать, что творится в глубинах тёмной сети? Blacksprut — это символ анонимности, скорости и безопасности, а не просто бренд. Посети bs2best.at и узнай то, о чём остальные предпочитают не говорить. Тебе откроют все тайны, скрытые от посторонних глаз. Только для посвящённых. Никаких следов. Никаких полумер. Только Blacksprut. Не упусти свой шанс быть впереди — bs2best.at ждёт тех, кто готов к новому. Осмелишься ли ты узнать истину?
Je suis emerveille par Casinia Casino, ca distille un plaisir eclatant. L’eventail de jeux est resplendissant, avec des slots aux designs audacieux et thematiques. 100% jusqu’a 500 € + 200 tours gratuits. Le suivi est irreprochable, toujours pret a eclairer. Les transactions sont fiables et rapides, cependant quelques tours gratuits supplementaires seraient lumineux. En bref, Casinia Casino garantit un plaisir radiant a chaque instant pour les aficionados de jeux modernes ! De plus la plateforme scintille comme une etoile, intensifie l’eclat du jeu. Egalement remarquable les evenements communautaires captivants, qui renforce l’engagement.
Accéder à l’avis|
Je suis captive par Casinia Casino, c’est une plateforme qui rayonne de luxe. La selection de jeux est royale, comprenant des jeux compatibles avec les cryptos. Amplifiant le plaisir de jeu. L’assistance est efficace et courtoise, offrant des reponses claires. Les retraits sont rapides comme l’eclair, neanmoins des recompenses additionnelles seraient royales. En resume, Casinia Casino vaut largement le detour pour les passionnes de jeux modernes ! En prime le site est rapide et attrayant, donne envie de prolonger l’experience. Particulierement interessant le programme VIP avec 5 niveaux, qui booste l’engagement.
Commencer ici|
J’adore l’ambiance de Locowin Casino, ca transporte dans un monde captivant. La selection de jeux est phenomenale, proposant des jeux de table immersifs. Pour un demarrage en force. Les agents repondent avec rapidite, avec une aide precise et rapide. Les retraits sont ultra-rapides, neanmoins plus de promos regulieres seraient top. En bref, Locowin Casino vaut largement le detour pour les amateurs de sensations fortes ! En prime la plateforme est visuellement top, amplifie le plaisir de jouer. Un autre atout majeur les paiements securises en crypto, assure des transactions fiables.
Continuer ici|
Флойд Мейвезердин ысымы бокс менен синоним болуп калган. Анын ар бир беттеши дүйнө жүзүнө көрсөтүлөт. Бокс легендасынын жетишкендиктери тууралуу окуу үчүн [url=https://floyd-mayweather-kg.com/]Флойд Мейвезер жетишкендиктер[/url] барагын караңыз.
Флойд Мейвезердин жашоосу мотивация берет. Анын карьерасы ар дайым жеңиш менен коштолгон. Анын рингдеги кыймылы ар дайым көрүүчүлөрдү суктандырат.
Флойд Мейвезердин акыркы жаңылыктары спорт дүйнөсүн дүрбөлөңгө салат.
Флойд Мейвезер ар дайым өз максатына жеткен. Флойд Мейвезердин жашоосу ар дайым элдин көңүлүндө. Флойд Мейвезер спорттун символу катары белгилүү.
Строительный сайт https://okna-k.com.ua для профессионалов и новичков. Новости отрасли, обзоры материалов, технологии строительства и ремонта, советы мастеров и пошаговые инструкции для качественного результата.
Сайт о металлах https://metalprotection.com.ua и металлообработке: виды металлов, сплавы, технологии обработки, оборудование и новости отрасли. Всё для специалистов и профессионалов металлургии.
Портал о дизайне https://sculptureproject.org.ua интерьеров и пространства. Идеи, тренды, проекты и вдохновение для дома, офиса и общественных мест. Советы дизайнеров и примеры стильных решений каждый день.
Главный автопортал страны https://nmiu.org.ua всё об автомобилях в одном месте! Новости, обзоры, советы, автообъявления, страхование, ТО и сервис. Для водителей, механиков и просто любителей машин.
I’m deeply intrigued by Wazamba Casino, it builds a thrilling narrative. The diversity in titles is astounding, tailored for cryptocurrency usage. Amplifying early engagement. Assistance team is superb. Financial dealings are protected and swift, even if regular deals might amplify appeal. To conclude, Wazamba Casino supplies premium leisure for quest pursuers ! Additionally the setup is accessible and evocative, infusing added allure. A further advantage exclusive ranks with special privileges, cultivating group dynamics.
wazambagr.com|
video talk
I adore the rush of Astronaut Crash by 100HP Gaming, it’s a pulse-racing voyage through the stars. Fair play is baked in with provable tech, with auto-bet for hands-free excitement. Bonus-free spins on select platforms. Help desk is responsive and expert, upholding crypto funding security. The exit system rescues bold bets, even so extra in-game power-ups would spice it up. All told, Astronaut Crash is essential for high-rollers for tactical thinkers ! Extra perk graphics pop with futuristic flair, streamlining stake tweaks. Game-changer practice arena with live data, sparks a vibrant player network.
https://astronaut-crashgame777.com/|
Студия ремонта https://anti-orange.com.ua квартир и домов. Выполняем ремонт под ключ, дизайн-проекты, отделочные и инженерные работы. Качество, сроки и индивидуальный подход к каждому клиенту.
Туристический портал https://feokurort.com.ua для любителей путешествий! Страны, маршруты, достопримечательности, советы и лайфхаки. Планируйте отдых, находите вдохновение и открывайте мир вместе с нами.
pronostics du foot 1xbet africain
1xbet cameroun apk telecharger 1xbet apk
telecharger 1xbet cameroun telecharger 1xbet cameroun
This is something that will need all of our combined efforts to address.
Флойд Мейвезер спорт дүйнөсүндөгү эң бай спортчу. Флойд Мейвезер эч качан жеңилбеген мушкер катары белгилүү. Жаңы жаңылыктарды окуу үчүн [url=https://floyd-mayweather-kg.com/]floyd-mayweather-kg.com[/url] барагына өтүңүз.
Флойд Мейвезер жаш кезинен тарта бокс менен алектенген. Анын рекорддору бүгүнкү күнгө чейин сакталган. Флойд Мейвезер ар дайым спорттун символу катары айтылат.
Флойд Мейвезердин акыркы жаңылыктары спорт дүйнөсүн дүрбөлөңгө салат.
Анын карьерасы ар бир жаш спортчу үчүн үлгү. Анын ар бир кадамы медиада талкууланат. Флойд Мейвезер ар дайым жаңы рекорддорду жарата берет.
spieler gegen spieler wette
Take a look at my website … online sportwetten Schweiz
online sportwetten Tipps für Heute
vergleich
Ich bin fasziniert von SpinBetter Casino, es bietet einen einzigartigen Kick. Der Katalog ist reichhaltig und variiert, mit innovativen Slots und fesselnden Designs. Der Support ist 24/7 erreichbar, garantiert top Hilfe. Die Transaktionen sind verlasslich, gelegentlich die Offers konnten gro?zugiger ausfallen. Global gesehen, SpinBetter Casino ist absolut empfehlenswert fur Adrenalin-Sucher ! Au?erdem die Interface ist intuitiv und modern, erleichtert die gesamte Erfahrung. Zusatzlich zu beachten die mobilen Apps, die den Einstieg erleichtern.
https://spinbettercasino.de/|
Je suis completement obsede par Locowin Casino, il delivre une experience unique. Le repertoire est opulent et multifacette, offrant des sessions live intenses. Pour un lancement puissant. Le service est operationnel 24/7, assurant un support premium. Les operations sont solides et veloces, bien que des bonus plus diversifies seraient souhaitables. En synthese, Locowin Casino garantit du divertissement constant pour les adeptes de sensations intenses ! En outre l’interface est intuitive et raffinee, ajoute un confort notable. Un plus significatif les options de paris sportifs etendues, offre des privileges sur mesure.
Locowin|
Студия дизайна https://bathen.rv.ua интерьеров и архитектурных решений. Создаём стильные, функциональные и гармоничные пространства. Индивидуальный подход, авторские проекты и внимание к деталям.
Ремонт и строительство https://fmsu.org.ua без лишних сложностей! Подробные статьи, обзоры инструментов, лайфхаки и практические советы. Мы поможем построить, отремонтировать и обустроить ваш дом.
Je suis completement ensorcele par Casinia Casino, c’est une plateforme qui scintille de grandeur. Le repertoire est riche et varie, comprenant des jeux compatibles avec les cryptos. Elevant l’experience de jeu. Le support client est royal, toujours pret a regner. Le processus est lisse et distingue, cependant plus de promos frequentes brilleraient davantage. Au final, Casinia Casino fournit une experience inoubliable pour les amateurs de casino en ligne ! De plus la navigation est intuitive comme un edit, ajoute une touche de splendeur. A souligner aussi les evenements communautaires engageants, qui renforce l’engagement.
Lire la version complГЁte|
Je suis impressionne par Casinia Casino, il offre une experience imperiale. La variete des titres est majestueuse, comprenant des jeux compatibles avec les cryptos. Amplifiant le plaisir de jeu. Le suivi est impeccable, avec une aide precise. Les paiements sont securises et fluides, bien que des bonus plus varies seraient apprecies. Pour conclure, Casinia Casino offre une experience memorable pour les fans de casino en ligne ! Notons aussi l’interface est intuitive et elegante, ajoute une touche de luxe. Un plus non negligeable les options de paris sportifs variees, offre des recompenses continues.
Visiter pour plus|
Je suis surpris par Locowin Casino, on detecte une vibe folle. Il y a une profusion de jeux captivants, proposant des jeux de table immersifs. Doublement des depots jusqu’a 1850 €. Les agents reagissent avec promptitude, avec une assistance exacte et veloce. Les benefices arrivent sans latence, bien que plus de promotions frequentes seraient un atout. En synthese, Locowin Casino merite une exploration approfondie pour les aficionados de jeux contemporains ! Ajoutons que la plateforme est esthetiquement remarquable, ajoute un confort notable. Un plus significatif le programme VIP avec des niveaux exclusifs, qui stimule l’engagement.
Visiter la page web|
was heißt quote bei ecken wetten Anbieter
connectwiththeworldnow – I appreciate how simple yet bold the site looks and speaks.
sportwetten deutscher anbieter
Also visit my page … wetten italien deutschland (Avis)
sportwetten systemwette strategie
Stop by my page; online Wette
What’s up everyone, it’s my first pay a quick visit at this site, and article is genuinely fruitful designed for me, keep up posting such articles.
https://kra41-cc.cc
I’m blown away by Wazamba Casino, it creates an adventurous aura. The library is truly enormous, including live casino for real-time action. Boosting your playtime. Support team is exceptional. Payouts are handled swiftly, at times extra spins might enhance it. Altogether, Wazamba Casino stands out as a superior platform for adventure seekers ! Additionally the platform loads quickly, infusing extra charm. Noteworthy is VIP tiers with exclusive privileges, building community engagement.
wazambagr.com|
wettformat gratiswette
Also visit my blog post :: sportwetten bonus angebote –
Ernie,
https://bs2tcite4.io
Современная студия дизайна https://bconline.com.ua архитектура, интерьер, декор. Мы создаём пространства, где технологии сочетаются с красотой, а стиль — с удобством.
foot africain afrik foot pronostic
parier pour le foot 1xbet cameroun apk
pronostic foot gratuit melbet – paris sportif
Создавайте дом https://it-cifra.com.ua своей мечты! Всё о строительстве, ремонте и дизайне интерьера. Идеи, проекты, фото и инструкции — вдохновляйтесь и воплощайте задуманное легко и с удовольствием.
Only reliable facts: https://www.jec.qa
We have reliable sources: https://justicelanow.org
Information you can trust: https://fgvjr.com
Only verified data: https://playplayfun.com
Only verified sources: https://www.leristrutturazioni.it
ascendgrid – Great name, hope their content or services match.
We only tell the facts: https://www.anak2u.com.my
A trusted source: https://www.feldbahn-ffm.de
Stay up to date: https://childstrive.org
No lies – just facts: https://www.acuam.com
We check every word: https://labhgroup.com
News as it is: https://www.bigcatalliance.org
Information you can trust: https://theshaderoom.com
We tell it like it is: https://nature-et-avenir.org
Only the latest updates: https://nusapure.com
Find out the truth here: https://jnp.chitkara.edu.in
truelaunch – Great mix of insight and usability, kudos to the creators.
brandvision – Very clean interface, made browsing feel effortless.
https://t.me/tripscan_1
We tell it like it is: https://www.forumishqiptar.com
Find out the truth here: https://anthese.fr
Only the latest updates: https://www.lagodigarda.com
News as it is: https://www.feldbahn-ffm.de
Мир архитектуры https://vineyardartdecor.com и дизайна в одном месте! Лучшие идеи, проекты и вдохновение для дома, офиса и города. Узнай, как создаются красивые и функциональные пространства.
электрокарнизы купить в москве [url=https://www.karniz-elektroprivodom.ru]электрокарнизы купить в москве[/url] .
согласование перепланировки нежилого помещения в москве [url=https://pereplanirovka-nezhilogo-pomeshcheniya10.ru]https://pereplanirovka-nezhilogo-pomeshcheniya10.ru[/url] .
стоимость. экскаватора. на. час. [url=http://arenda-mini-ekskavatora-v-moskve-2.ru/]http://arenda-mini-ekskavatora-v-moskve-2.ru/[/url] .
kombiwetten heute
my webpage :: WettbüRo Aachen
перепланировка нежилого здания [url=http://pereplanirovka-nezhilogo-pomeshcheniya11.ru]перепланировка нежилого здания[/url] .
live broadcast
Ich habe einen totalen Hang zu SpinBetter Casino, es liefert ein Abenteuer voller Energie. Das Angebot an Spielen ist phanomenal, mit dynamischen Tischspielen. Der Service ist von hoher Qualitat, mit praziser Unterstutzung. Die Transaktionen sind verlasslich, gelegentlich die Offers konnten gro?zugiger ausfallen. In Kurze, SpinBetter Casino ist ein Muss fur alle Gamer fur Adrenalin-Sucher ! Hinzu kommt das Design ist ansprechend und nutzerfreundlich, was jede Session noch besser macht. Ein weiterer Vorteil die Sicherheit der Daten, die das Spielen noch angenehmer machen.
https://spinbettercasino.de/|
We select the best: https://www.greenwichodeum.com
Only important details: https://theshaderoom.com
Time-tested and proven: https://www.amakmeble.pl
Information you can trust: https://satapornbooks.com
J’ai une passion debordante pour Locowin Casino, c’est une plateforme qui deborde de vitalite. La diversite des titres est stupefiante, avec des slots innovants et thematises. Le bonus d’accueil est seduisant. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les gains arrivent sans retard, de temps en temps quelques tours gratuits supplementaires seraient bienvenus. Au final, Locowin Casino est une plateforme qui impressionne pour ceux qui aiment parier en crypto ! En bonus l’interface est intuitive et elegante, facilite une immersion totale. Un autre point fort les paiements securises en crypto, qui booste l’engagement.
Découvrir l’histoire complète|
согласование перепланировки нежилого помещения в москве [url=http://www.pereplanirovka-nezhilogo-pomeshcheniya9.ru]http://www.pereplanirovka-nezhilogo-pomeshcheniya9.ru[/url] .
J’ai un engouement sincere pour Locowin Casino, ca transporte dans un univers envoutant. Il y a une profusion de jeux captivants, comprenant des jeux adaptes aux cryptos. Doublement des depots jusqu’a 1850 €. L’aide est performante et experte, proposant des reponses limpides. Les retraits sont realises promptement, mais des offres plus liberales ajouteraient de la valeur. En fin de compte, Locowin Casino merite une exploration approfondie pour les aficionados de jeux contemporains ! Par ailleurs le site est veloce et seduisant, intensifie le plaisir du jeu. Egalement notable les options de paris sportifs etendues, garantit des transactions securisees.
Voir les meilleurs choix|
J’ai un veritable engouement pour Locowin Casino, c’est une plateforme qui explose d’energie. La diversite des titres est epoustouflante, comprenant des jeux adaptes aux cryptos. Doublement des depots jusqu’a 1850 €. Le suivi est exemplaire, accessible a tout instant. Les operations sont solides et veloces, de temps a autre des incitations additionnelles seraient un benefice. Pour synthetiser, Locowin Casino merite une exploration approfondie pour ceux qui parient en crypto ! De surcroit le site est veloce et seduisant, intensifie le plaisir du jeu. Un plus significatif le programme VIP avec des niveaux exclusifs, garantit des transactions securisees.
Poursuivre la lecture|
valuevision – I like the color scheme, it gives a calming vibe.
рулонные шторы в москве [url=https://rulonnaya-shtora-s-elektroprivodom.ru]рулонные шторы в москве[/url] .
alihidaer2313 – I enjoyed browsing, the feel is straightforward and uncluttered.
growthverse – The site is well-structured, making information easy to find.
I have a real passion for Wazamba Casino, it delivers a unique rush. The options are extensive and diverse, including slots with tribal themes. The welcome bonus is generous. Agents respond swiftly. Transactions are hassle-free, sometimes additional bonuses might be welcome. All in all, Wazamba Casino delivers top-tier gaming for online betting fans ! Moreover the site is fast and responsive, encourages repeated visits. Another perk is crypto-friendly banking options, offering more ways to win.
https://wazambagr.com/|
skyportal – Articles are informative, I keep coming back every week.
Γεια χαρά σε όλους! Εδώ είμαστε πάλι, η ομάδα των ειδικών, για να βουτήξουμε σε ένα θέμα που καίει πολλούς παίκτες στην Ελλάδα: ποιές ξένες στοιχηματικές εταιρίες δέχονται τους παίκτες από την χώρα μας ελεύθερα, χωρίς VPN και γρήγορες πληρωμές; Έχουμε περάσει ώρες ατελείωτες δοκιμάζοντας διάφορες πλατφόρμες τζόγου, διαβάζοντας ψιλά γράμματα και μιλώντας με ομάδα υποστήριξης, για να σας δώσουμε την ξεκάθαρη εικόνα για το τι παίζει σε αυτές τις πλατφόρμες πραγματικά.
trendforge – The name suggests being ahead of trends; that’s appealing.
Fiquei impressionado com BETesporte Casino, proporciona uma aventura competitiva. Ha uma multidao de jogos emocionantes, com sessoes ao vivo imersivas. O bonus de boas-vindas e empolgante. Os agentes respondem com velocidade, com suporte preciso e rapido. As transacoes sao confiaveis, contudo bonus mais variados seriam um gol. No fim, BETesporte Casino e uma plataforma que domina o campo para jogadores em busca de emocao ! Adicionalmente o design e moderno e dinamico, instiga a prolongar o jogo. Muito atrativo os torneios regulares para rivalidade, que impulsiona o engajamento.
Ler mais|
Only verified information: https://fasterskier.com
Only verified facts: https://agriness.com
Only real facts: https://casinocompendium.com
We write as is: https://petitedanse.com.br
Trustworthy news: https://gingerparrot.co.uk
quantumreach – Overall a positive experience, I’ll be returning for more.
boldimpact – Clean branding, simple but memorable name.
alphaunity – Great mix of short quick reads and in-depth content.
A source for current events: https://kariyanichigeki.com
Events without embellishment: https://possible11.com
No exaggerations: https://tennews.in
Source of truthful news: https://www.chronicle.ng
Only real events: https://cfsl.in
bluetrail – Found several ideas I hadn’t thought of before, nice.
sharpbridge – Navigation feels intuitive, didn’t struggle finding anything so far.
solidvision – Hoping they launch soon; curious what they’ll offer.
elitegrowth – The site is well-structured, making information easy to find.
cloudmatrix – Consistently impressed with the site’s performance and design.
goldbridge – Impressed by fast loading and responsiveness on mobile.
We maintain objectivity: https://institutocea.com
Все лучшее у нас: https://familylab-spa.ru
We value honesty: https://alterego.re
Все самое актуальное тут: https://rr-game.ru
No fakes or speculation: https://livescores.biz
fastgrowth – Consistently impressed with the site’s performance and design.
maxvision – Impressed by the layout, feels modern and user-friendly.
Автоматические гаражные ворота давно перестали быть роскошью и стали необходимым элементом комфортной жизни. Наши автоматические ворота сочетают надёжность проверенных европейских механизмов с элегантным дизайном, который гармонично впишется в архитектуру любого здания. Мы предлагаем полный цикл услуг: от профессиональной консультации и точного замера до установки под ключ и гарантийного обслуживания. Доверьте безопасность своего дома профессионалам — получите бесплатный расчёт стоимости уже сегодня: Подробнее
greenmotion – Offers valuable insights, and the interface is top-notch.
zenithlabs – The content is engaging, and the site is responsive.
thinkbeyondtech – Great user experience, everything loads so smoothly and fast.
nextlayer – Impressed by the layout, feels modern and user-friendly.
Заботьтесь о здоровье сосудов ног с профессионалом! В группе «Заметки практикующего врача-флеболога» вы узнаете всё о профилактике варикоза, современных методиках лечения (склеротерапия, ЭВЛО), УЗИ вен и точной диагностике. Доверяйте опытному врачу — ваши ноги заслуживают лучшего: https://phlebology-blog.ru/
boldnetwork – Hoping they’ll add more info soon; I like the design direction.
https://bs2site4.io
Всё самое свежее здесь: https://actual-cosmetology.ru
Только проверенные новости: https://fontanvazon.ru
Всё актуальное в одном месте: https://radiocert.ru
Всё самое новое тут: https://forwardprint.com.ua
Источник, которому доверяют: https://school33-perm.ru
wettanbieter mit bonus
my webpage :: beste quoten online sportwetten (http://Www.bofan-cable.com)
halbzeit endergebnis wetten
Also visit my web page: gratiswette ohne einzahlung sofort
J’adore l’ambiance de Locowin Casino, il offre une experience unique. Il y a une multitude de jeux excitants, incluant des paris sportifs palpitants. Le bonus de bienvenue est attractif. Le support client est exceptionnel, joignable a toute heure. Les transactions sont fiables et rapides, de temps en temps quelques tours gratuits en plus seraient cool. Pour conclure, Locowin Casino offre une experience memorable pour les joueurs en quete d’excitation ! Notons aussi l’interface est intuitive et stylee, ajoute une touche de confort. Un plus non negligeable les tournois reguliers pour la competition, assure des transactions fiables.
Visiter la page web|
J’ai un engouement sincere pour Locowin Casino, on detecte une vibe folle. La diversite des titres est epoustouflante, proposant des jeux de table immersifs. Renforcant l’experience de depart. L’equipe d’assistance est remarquable, toujours pret a intervenir. Les retraits sont realises promptement, par moments quelques tours gratuits supplementaires seraient apprecies. Globalement, Locowin Casino merite une exploration approfondie pour les joueurs a la recherche d’aventure ! Ajoutons que l’interface est intuitive et raffinee, facilite une immersion complete. Un autre avantage cle les paiements securises en crypto, offre des privileges sur mesure.
Voir tout|
J’aime l’atmosphere de Locowin Casino, on detecte une vibe folle. Le repertoire est opulent et multifacette, proposant des jeux de table immersifs. Renforcant l’experience de depart. Le service est operationnel 24/7, assurant un support premium. Les benefices arrivent sans latence, de temps a autre des bonus plus diversifies seraient souhaitables. Globalement, Locowin Casino est essentiel pour les amateurs pour ceux qui parient en crypto ! Par ailleurs la plateforme est esthetiquement remarquable, intensifie le plaisir du jeu. Particulierement attractif les tournois periodiques pour la rivalite, propose des recompenses permanentes.
VГ©rifier ceci|
ynaix – Wow this site looks slick, very modern and impressive visuals.
велотренажер магнитный Электрическая беговая дорожка складная — решение для малогабаритных квартир. Благодаря механизму складывания она занимает минимум места после использования, легко убирается под кровать или в шкаф. Мотор 1,5-2 л.с. обеспечивает скорость 1-16 км/ч, наклон 0-12%, с плавным стартом. Полотно с амортизацией 40×120 см комфортно для бега и ходьбы, а сенсоры пульса на рукоятках мониторят сердцебиение. Модели с Bluetooth синхронизируются с фитнес-приложениями для виртуальных маршрутов. Вес до 50 кг, нагрузка 100-120 кг. Идеальна для семейного использования. Покупка обойдется в 25 000-60 000 рублей. Складная дорожка способствует регулярным занятиям, улучшая выносливость и сжигая до 500 калорий за час.
deutsche sportwetten system strategie lizenz
bs2best at Готов узнать, что творится в глубинах тёмной сети? Blacksprut — это символ анонимности, скорости и безопасности, а не просто бренд. Посети bs2best.at и узнай то, о чём остальные предпочитают не говорить. Тебе откроют все тайны, скрытые от посторонних глаз. Только для посвящённых. Никаких следов. Никаких полумер. Только Blacksprut. Не упусти свой шанс быть впереди — bs2best.at ждёт тех, кто готов к новому. Осмелишься ли ты узнать истину?
connectwiththeworldnow – Overall experience is positive, site feels purposeful and authentic.
Надёжный источник информации: https://motorradhof.ru
Узнавайте события первыми: https://www.foto4u.su
Только проверенные факты: https://neoko.ru
hunderennen online wetten test (Edgardo) wetten
Частный заем денег домашние деньги кабинет альтернатива банковскому кредиту. Быстро, безопасно и без бюрократии. Получите нужную сумму наличными или на карту за считанные минуты.
strongrod – Typography is clean; reading is comfortable even on small screens.
Все спортивные новости http://sportsat.ru в реальном времени. Итоги матчей, трансферы, рейтинги и обзоры. Следите за событиями мирового спорта и оставайтесь в курсе побед и рекордов!
wavefusion – Found useful content, everything seems well organized.
brightideaweb – Overall nice polish, site feels professional and thoughtfully designed.
Me encantei pelo ritmo de BR4Bet Casino, tem um ritmo de jogo que acende como uma tocha. Os jogos formam um clarao de entretenimento. oferecendo lives que acendem como fogueiras. Os agentes voam como claroes. com solucoes precisas e instantaneas. As transacoes sao faceis como um brilho. ocasionalmente mais recompensas fariam o coracao brilhar. No geral, BR4Bet Casino garante um jogo que reluz como um farol para os fas de adrenalina brilhante! Adicionalmente o design e fluido como uma lanterna. criando uma experiencia de cassino reluzente.
br4bet rollover|
Je suis fou de Simsinos Casino, offre un spectacle de plaisir qui dessine. vibre avec un storyboard de jeux varies. incluant des jeux de table de casino d’une elegance cartoon. Le support du casino est disponible 24/7. joignable par chat ou email. Le processus du casino est transparent et sans fondu. tout de meme des offres qui vibrent comme une cadence animee. Globalement, Simsinos Casino cadence comme une sonate de victoires pour les explorateurs de melodies en ligne! A noter le site du casino est une merveille graphique coloree. ce qui rend chaque session de casino encore plus animee.
simsinos casino review|
metarise – Really enjoying the look, everything feels modern and smooth.
Amo a energia de BETesporte Casino, sinto um rugido de emocao. A variedade de titulos e impressionante, com slots modernos e tematicos. 100% ate R$600 + apostas gratis. O acompanhamento e impecavel, oferecendo respostas claras. O processo e simples e direto, de vez em quando recompensas extras seriam um hat-trick. Em sintese, BETesporte Casino vale uma aposta certa para amantes de apostas esportivas ! Vale destacar a plataforma e visualmente impactante, aumenta o prazer de apostar. Um diferencial importante os pagamentos seguros em cripto, fortalece o senso de comunidade.
Saiba mais|
cyberlaunch – Useful resources are easy to access, menus make sense.
Galera, preciso compartilhar minha experiencia no 4PlayBet Casino porque me pegou de surpresa. A variedade de jogos e surreal: poquer estrategico, todos com graficos de primeira. O suporte foi bem prestativo, responderam em minutos pelo chat, algo que passa seguranca. Fiz saque em PIX e o dinheiro entrou sem enrolacao, ponto fortissimo. Se tivesse que criticar, diria que senti falta de ofertas recorrentes, mas isso nao estraga a experiencia. No geral, o 4PlayBet Casino e completo. Eu ja voltei varias vezes.
4play 4p55|
sharpwave – Love the crisp styling, makes everything look polished and clean.
Sou viciado em PlayPIX Casino, leva a um universo de pura adrenalina. O catalogo e rico e multifacetado, suportando jogos compativeis com criptomoedas. Com uma oferta inicial para impulsionar. Os agentes respondem com agilidade, oferecendo respostas claras. As transacoes sao confiaveis, as vezes promocoes mais frequentes dariam um toque extra. No geral, PlayPIX Casino garante diversao constante para entusiastas de jogos modernos ! Alem disso a plataforma e visualmente espetacular, adiciona um toque de conforto. Outro destaque os torneios regulares para competicao, fortalece o senso de comunidade.
Ler mais|
brightchain – Color scheme works, contrasts help readability without being harsh.
innovatewithus – Content is super useful, I learned something new today.
cloudmark – The homepage is elegant, feels welcoming and well-designed.
nextbrand – Visuals work well, colors complement each other nicely.
digitalstorm – Typography sharp, spacing helps readability significantly.
everythingyouneedtoknow – Writing is crisp, not dragging, gives value quickly.
discoveramazingthingsonline – Layout is playful but not messy, balance is just right.
explorecreativeideasdaily – Visuals are engaging, complement the creative topics perfectly.
микрозайм онлайн
Понадобилось сделать ремонт в кабинете, не хватило небольшой суммы. Решил взять займ здесь. Мне одобрили заявку за несколько минут. Оформляю микрозайм онлайн, и деньги сразу на карте. Никаких процентов, все честно. Очень доволен, спасибо.
https://bs2site.gdn
ultrawave – The voice feels genuine, not trying too hard to impress.
pixelplanet – Pages load fast; images crisp even on mobile usage.
yourbrandzone – Overall very polished; gives confidence in what’s offered here.
Доставка пиццы Воронеже https://pizzeriacuba.ru самая вкусная сочная пицца в городе Воронеж. Доставим пиццу горячей круглочуточно в Воронеже скидки от 3500 рублей. Скидка на самовывоз на каждую пиццу. Доставка бесплатно
zenithmedia – The tone feels trustworthy and knowledgeable without being overbearing.
boldspark – Really love the punchy design, makes a strong first impression.
Suchen Sie Immobilien? immobilien in Montenegro wohnungen, Villen und Grundstucke mit Meerblick. Aktuelle Preise, Fotos, Auswahlhilfe und umfassende Transaktionsunterstutzung.
joinourcreativecommunity – Typography is friendly and readable, spacing makes it easygoing.
Je suis totalement fou de Locowin Casino, ca transporte dans un monde captivant. La variete des titres est impressionnante, incluant des paris sportifs palpitants. Amplifiant l’experience initiale. Le support client est exceptionnel, joignable a toute heure. Les retraits sont ultra-rapides, parfois des offres plus genereuses ajouteraient du piquant. En resume, Locowin Casino est une plateforme qui dechire pour les passionnes de jeux modernes ! De plus la plateforme est visuellement top, donne envie de prolonger l’experience. Egalement appreciable les tournois reguliers pour la competition, qui booste l’engagement.
Tout trouver|
YourPathOfGrowth – Their approach to education is both innovative and impactful.
Je suis emerveille par Casinia Casino, ca transporte dans un palais scintillant. Les options sont vastes comme un ciel etoile, avec des slots aux designs audacieux et thematiques. Avec un Bonus Crab unique pour debuter. L’assistance est rapide et precise, joignable a toute heure. Les paiements sont securises et fluides, neanmoins des offres plus genereuses seraient un plus. En somme, Casinia Casino est un joyau pour les joueurs pour les joueurs en quete d’eclat ! A noter le site est rapide et envoutant, ajoute une touche de splendeur. Un atout cle le programme VIP avec 5 niveaux exclusifs, cree une communaute vibrante.
Tout apprendre|
Je suis epate par Locowin Casino, il offre une experience unique. Le catalogue est riche et diversifie, comprenant des jeux compatibles avec les cryptos. Accompagne de tours gratuits sans wager. Le suivi client est irreprochable, offrant des reponses claires. Les retraits sont ultra-rapides, parfois des recompenses supplementaires seraient un atout. Dans l’ensemble, Locowin Casino offre une experience memorable pour les passionnes de jeux modernes ! De plus la navigation est simple et agreable, donne envie de prolonger l’experience. Particulierement interessant les paiements securises en crypto, renforce le sentiment de communaute.
Obtenir les bonus|
LearnWithConfidence – The community feedback is excellent, makes learning feel interactive and fun.
alphaimpact – Overall very polished, site feels professional and trustworthy.
makingeverymomentcount – The name itself already motivates, content backs it up too.
thebestplacetostarttoday – The tone speaks to encouragement, not pressure, very refreshing.
learnshareandsucceed – Navigation is clear, I easily found a section I liked.
getreadytoexplore – The tone is curious and welcoming, makes me want to stay longer.
Sou louco pela matriz de PlayPix Casino, explode com uma vibe de cassino retro-futurista. As escolhas sao vibrantes como um glitch. com jogos adaptados para criptomoedas. O suporte e um firewall de eficiencia. respondendo rapido como um buffer. Os saques sao velozes como um upload. mesmo assim mais bonus regulares seriam digitais. No fim das contas, PlayPix Casino garante um jogo que reluz como pixels para os hackers do cassino! Adicionalmente o design e um espetaculo visual de matriz. tornando cada sessao ainda mais pixelada.
playpix]|
everythingaboutsuccess – Inspiring site, content gives me real motivation to push forward.
findwhatyouarelookingfor – Typography and spacing are comfortable, reading feels effortless.
Acho simplesmente ardente Fogo777 Casino, parece um festival de chamas cheio de adrenalina. O catalogo de jogos e um altar de prazeres. com slots tematicos de cerimonias antigas. O time do cassino e digno de um guardiao do fogo. com ajuda que ilumina como uma pira. Os ganhos chegam rapido como uma labareda. ocasionalmente queria mais promocoes que queimam como fogueiras. No fim das contas, Fogo777 Casino vale explorar esse cassino ja para os fas de adrenalina ardente! Como extra o layout e vibrante como uma tocha. adicionando um toque de fogo ao cassino.
jogo fogo777|
learnsomethingneweveryday – Love this idea, content always surprises me with new insights.
discoverendlessinspiration – Navigation flows naturally, discovering content is a treat.
growyourbusinessfast – Found actionable tips right away, no filler content.
A vibrant platformer guiding a panda through exciting puzzles and treasures. Fun and family-friendly: Panda puzzle games for free
Estou completamente encantado com BETesporte Casino, oferece um prazer esportivo inigualavel. Ha uma explosao de jogos emocionantes, com sessoes ao vivo cheias de energia. Com uma oferta inicial para impulsionar. O acompanhamento e impecavel, garantindo atendimento de alto nivel. O processo e simples e direto, embora ofertas mais generosas seriam bem-vindas. Para finalizar, BETesporte Casino oferece uma experiencia inesquecivel para entusiastas de jogos modernos ! Alem disso a navegacao e intuitiva e rapida, instiga a prolongar o jogo. Notavel tambem os eventos comunitarios envolventes, proporciona vantagens personalizadas.
Descobrir|
makeimpactwithideas – Helpful tools and sections make it worthwhile to stay and explore.
Fiquei fascinado com PlayPIX Casino, oferece um prazer intenso e indomavel. A variedade de titulos e estonteante, com sessoes ao vivo cheias de energia. O bonus de boas-vindas e cativante. O servico esta disponivel 24/7, sempre pronto para resolver. Os saques sao rapidos como um raio, no entanto bonus mais variados seriam incriveis. Em sintese, PlayPIX Casino vale uma visita epica para jogadores em busca de adrenalina ! Adicionalmente a plataforma e visualmente espetacular, instiga a prolongar a experiencia. Igualmente impressionante os pagamentos seguros em cripto, assegura transacoes confiaveis.
Ver os detalhes|
https://monopoly-dice.com/welcome-to-monopoly-dice/
SimpleWaysToBeHappy – A great reminder to find joy in the little things.
BuildSomethingMeaningful – This platform is a valuable tool for community leaders.
GrowWithConfidenceHere – I appreciate how structured and supportive the courses are here.
YourTrustedSourceOnline – It’s inspiring to see how they empower local communities.
TogetherWeCreateChange – Navigation is smooth, finding resources for projects is super fast.
BuildABetterTomorrow – I appreciate how they focus on sustainable solutions for all.
CreateInspireAndGrow – The resources provided are incredibly helpful for anyone looking to improve.
sportwetten lizenz deutschland beantragen
Also visit my blog post – wett tipp heute (Monica)
stayupdatedwithtrends – The tone is confident and helpful, not too buzzwordy.
pferderennen iffezheim wetten
Feel free to visit my homepage … Esc buchmacher quoten
женский фитнес клуб в москве https://fitnes-klub-msk.ru
женский фитнес клуб в москве фитнес клуб тренировка
Гарри Кейн ар дайым максатка умтулат.Кейн дайыма жоопкерчиликти өз мойнуна алат. Футбол күйөрмандары үчүн ишенимдүү булак — [url=https://harry-kane-kg.com/]https://harry-kane-kg.com/[/url]. Кейн бир нече жолу “Алтын бутса” уткан.
Кейндин айлык акысы чоң талкууга себеп болду.
Гарри Кейн жеке жашоосун ачык көрсөтпөйт. Бул оюнчу коргонууда да пайдалуу. Бул оюнчу эч качан берилишпейт. Кейндин алгачкы командалары ага чоң тажрыйба берген. Бул оюнчу кайсы чемпионатта болбосун айырмаланат. Кейндин трансферлери ар дайым чоң темалар.
TurnIdeasIntoAction – I appreciate how they encourage doing rather than just dreaming.
EverythingStartsWithYou – I love the empowering messages here, feels like personal encouragement.
pferderennen wetten regeln
Feel free to visit my blog post :: Wett Tipps Von Profis
https://b2tsite4.io
beste deutsche wettanbieter
Here is my page: Sportwetten tipps Erfahrung
LearnSomethingAwesome – The community comments add extra ideas I hadn’t considered.
FindTheBestIdeas – Definitely bookmarking this for when I need a creative boost or spark.
Кейндин упай топтоо жөндөмү легендардуу.Ар бир оюну күйөрмандар үчүн майрам. Бул сайтта Кейндин толук статистикасы жана оюн жыйынтыктары бар: [url=https://harry-kane-kg.com/]Гарри Кейн статистикасы[/url]. Гарри Кейндин жыйнаган трофейлери улам көбөйүп жатат.
Гарри Кейн Германияда да өз деңгээлин көрсөттү.
Гарри Кейн жеке жашоосун ачык көрсөтпөйт. Бул оюнчу коргонууда да пайдалуу. Анын ар бир голу эстен чыкпайт. Кейндин футболго болгон сүйүүсү жаш кезинен башталган. Ал ар бир сезон ондогон гол киргизет. Кейндин трансферлери ар дайым чоң темалар.
LearnShareAndGrow – Their commitment to social impact is truly commendable.
https://www.mundus-online.de/faq-items/donec-ac-iaculis-lorem-sit-venenatis-tellus/
online wettanbieter deutschland
Stop by my page; buchmacher angebote, keyweb-264.dev.boosthost.net,
Сериалы 2024 скачать торрент Скачать фильмы торрент Погрузитесь в захватывающий мир кино, не выходя из дома, с помощью торрентов! На нашем сайте вы найдете огромный выбор фильмов на любой вкус: от умопомрачительных блокбастеров и трогательных мелодрам до захватывающих триллеров и интеллектуальных драм. Мы предлагаем загрузку фильмов в высоком качестве, включая HD, Full HD и даже 4K, чтобы вы могли наслаждаться каждой деталью на своем экране. У нас вы найдете как новинки кинопроката, так и проверенные временем классические фильмы. Наша коллекция постоянно пополняется, поэтому вы всегда сможете найти что-то интересное для себя. Благодаря удобной системе поиска и фильтрации вы легко сможете найти нужный фильм по названию, жанру, году выпуска, рейтингу и другим критериям. Скачивайте фильмы торрент быстро и безопасно с нашего сайта! Мы гарантируем высокое качество файлов и отсутствие вирусов. Наслаждайтесь просмотром любимых фильмов в любое время и в любом месте!
TheArtOfLivingWell – I appreciate the balance between motivation and gentle encouragement throughout.
FindNewWaysToGrow – Very motivating content, helps me push past my comfort zone.
FindAnswersAndIdeas – The community comments are just as insightful as the articles themselves.
Кейндин упай топтоо жөндөмү легендардуу.Бул оюнчу дайыма эң мыктылардын арасында. Эгер футбол сага жакса, анда бул шилтемени сөзсүз ачып көр: [url=https://harry-kane-kg.com/]Гарри Кейн жаңылыктары</url]. Гарри Кейн дайыма өз командасына ишеним жаратат.
Кейндин “Бавариядагы” карьерасы жаңы барак ачты.
Кейндин жашоосу көптөр үчүн үлгү. Кейндин карьерасы узак жана ийгиликтүү. Кейн ар бир сезон өзүн мыкты формада кармайт. Кейндин футболго болгон сүйүүсү жаш кезинен башталган. Бул оюнчу кайсы чемпионатта болбосун айырмаланат. Футбол сүйүүчүлөрү анын жаңылыктарын күтүшөт.
YourDigitalDestination – Helpful insights, nice to see such thoughtful explanations.
Гарри Кейн оюн талаасында дайыма тынчсызданууну жаратат.Футбол тарыхында анын аты чоң орунда болот. Футбол күйөрмандары үчүн ишенимдүү булак — [url=https://harry-kane-kg.com/]https://harry-kane-kg.com/[/url]. Бул чабуулчу ар бир мелдеште гол киргизүүгө даяр.
Кейндин айлык акысы чоң талкууга себеп болду.
Кейндин үй-бүлөсү ар дайым анын жанында. Кейн командалаштарын дайыма колдойт. Анын ар бир голу эстен чыкпайт. Анын жолун уланткандар көп. Ар бир күйөрман анын статистикасын сыймыктануу менен айтат. Кейн социалдык тармактарда активдүү.
GetConnectedWithPeople – Real conversations happen here, not just superficial chatter or posts.
UnlockYourPotentialToday – The design is clean and the pages load fast, love it.
FindSolutionsForLife – Bookmarking this, it’s going to be my go-to when I need answers.
ConnectDiscoverAndGrow – Love this platform, will be returning often for new ideas.
все самое лучшее тут: https://clinicasadelgazamiento.es/claves-efectivas-estrategias-para-mantener-un-peso-saludable/
DiscoverWorldOfIdeas – The posts are thoughtful, give me something to mull over later.
madeleinemtbc – Content here is rich, speaks directly to community heart
Кейндин оюндары дайыма эмоция тартуулайт.Кейн дайыма жоопкерчиликти өз мойнуна алат. Бул шилтемеде Гарри Кейндин оюндарынан эң мыкты учурлар чогулган: [url=https://harry-kane-kg.com/]Гарри Кейн видео[/url]. Футбол дүйнөсү мындай оюнчуларды сейрек көрөт.
Баварияда Кейн жаңы рекорддорду коюуда.
Кейндин үй-бүлөсү ар дайым анын жанында. Кейндин карьерасы узак жана ийгиликтүү. Анын ар бир голу эстен чыкпайт. Кейндин футболго болгон сүйүүсү жаш кезинен башталган. Бул оюнчу кайсы чемпионатта болбосун айырмаланат. Кейндин реал мадридге өтүшү дагы эле талкууда.
https://www.business-gazeta.ru/article/658582
markmackenzieforcongress – I keep coming back here for thoughtful insights and realistic ideas.
FindInspirationEverywhere – It’s a go-to when I feel stuck and need a spark.
YourGuideForSuccess – I appreciate the balance of motivation and real actionable steps offered here.
StartChangingYourLife – Layout is calm, reading feels soothing even when topics are serious.
DiscoverUsefulTipsDaily – The community comments often add helpful pointers I hadn’t considered.
DiscoverNewOpportunities – I appreciate how they focus on sustainable solutions for all.
BuildYourDreamFuture – There’s something uplifting about reading here after a rough day.
Сколько стоит аренда автобуса https://povozkin.ru
wett bild sportwetten tipps (education.k-lad.com) ergebnisse
blsp at Что, еще не слышал о сенсации, сотрясающей глубины даркнета? Blacksprut – это не просто торговая марка. Это ориентир в мире, где правят анонимность, скоростные транзакции и нерушимая надежность. Спеши на bs2best.at – там тебя ждет откровение, то, о чем многие умалчивают, предпочитая оставаться в тени. Ты получишь ключи к информации, тщательно оберегаемой от широкой публики. Только для тех, кто посвящен в суть вещей. Полное отсутствие следов. Никаких компромиссов. Лишь совершенство, имя которому Blacksprut. Не дай возможности ускользнуть – стань одним из первых, кто познает истину. bs2best.at уже распахнул свои объятия. Готов ли ты к встрече с реальностью, которая шокирует?
компания потолочкин [url=https://www.stretch-ceilings-nizhniy-novgorod.ru]https://www.stretch-ceilings-nizhniy-novgorod.ru[/url] .
**mindvault**
mindvault is a premium cognitive support formula created for adults 45+. It’s thoughtfully designed to help maintain clear thinking
makethemostoflife – I’ll keep this bookmarked, very uplifting resource
makeprogressdaily – The tips shared here are practical and easy to implement.
https://bs2site.cat
Анын оюндары Англия футболунун сыймыгы.Ар бир сезон Гарри Кейн жаңы деңгээлге чыгат. Атактуу англиялык чабуулчу тууралуу көбүрөөк билүү үчүн кир: [url=https://harry-kane-kg.com/]https://harry-kane-kg.com/[/url]. Кейн өзүнүн максаттарын так билет.
Кейн менен Бавария дагы күчтүү болуп калды.
Анын жубайы Кэти Гудленд аны колдойт. Кейн капитан катары чоң жоопкерчилик алат. Футбол дүйнөсүндө Гарри Кейн өз ордун тапкан. Кейндин алгачкы командалары ага чоң тажрыйба берген. Ал ар бир сезон ондогон гол киргизет. Гарри Кейндин акыркы жаңылыктары кызыктуу.
Лазерные станки https://raymark.ru для резки металла в Москве. 20 лет на рынке, выгодная цена, скидка 5% при заявке с сайта + обучение
HOME CLIMAT https://homeclimat36.ru кондиционеры и сплит системы в Воронеже. Скидка на монтаж от 3000 рублей! При покупке сплит-системы.
inspireandgrowtogether – The posts are insightful, offering fresh perspectives.
findthebestcontent – The layout is neat, not too many distractions or clutter
findyourwayforward – The message gives hope and a clearer path, very nice
discoverlearnandshare – Found something new I hadn’t seen elsewhere — neat find
опытный таролог Финансовый расклад таро Финансовый расклад таро – это специализированный расклад карт Таро, предназначенный для анализа финансовой ситуации и прогнозирования финансовых перспектив. Он помогает понять, откуда приходят деньги, куда они уходят, какие возможности существуют для увеличения дохода и как избежать финансовых проблем. Финансовый расклад таро может ответить на такие вопросы, как: Какова текущая финансовая ситуация? Какие факторы влияют на финансовую стабильность? Какие возможности существуют для увеличения дохода? Какие риски следует учитывать при принятии финансовых решений? Каковы перспективы инвестиций? Как улучшить финансовое планирование? Финансовый расклад таро помогает увидеть финансовую ситуацию с другой стороны, понять скрытые факторы и принять взвешенные решения, направленные на улучшение финансового благосостояния.
licsupport – I bookmark this, very helpful site for policy support
connectwithbrilliantminds – Great resources shared, feels like a smart community forming
everythingaboutmarketing – Excellent insights on modern marketing strategies, always up-to-date.
discoveryourpassion – This site really helped me find my true calling.
discovertrendingideas – The latest posts are really on point, I’m impressed
learnandearndaily – The writing feels genuine, not too salesy or pushy
connectandgrowonline – The integration with social media platforms is seamless and efficient.
jointhenextbigthing – Found some amazing tips, very helpful for beginners like me.
learnexploreandshine – Browsing feels fluid, I didn’t feel lost anywhere
togetherwecreateimpact – I’ll be coming back to see what’s next and engage more
findyourinspirationhere – Posts feel honest, like someone sharing their journey openly
yourgatewaytosuccess – I like the call-to-action style, makes me want to explore more
Нужна недвижимость? недвижимость Черногории купить лучшие объекты для жизни и инвестиций. Виллы, квартиры и дома у моря. Помощь в подборе, оформлении и сопровождении сделки на всех этапах.
Аутстаффинг персонала https://skillstaff2.ru для бизнеса: легальное оформление сотрудников, снижение налоговой нагрузки и оптимизация расходов. Работаем с компаниями любого масштаба и отрасли.
keepgrowingwithus – Love the vibe, feels like a place for real growth and progress
Estou alucinado com BR4Bet Casino, parece um festival de luzes cheio de adrenalina. As escolhas sao vibrantes como um farol. incluindo jogos de mesa com um toque de brilho. O atendimento e solido como um farol. disponivel por chat ou e-mail. Os pagamentos sao lisos como uma chama. mas mais giros gratis seriam uma loucura iluminada. Para encurtar, BR4Bet Casino e um cassino online que e um farol de diversao para os fas de adrenalina brilhante! Vale dizer o design e fluido como uma lanterna. elevando a imersao ao nivel de uma fogueira.
br4bet demora quanto tempo para cair na conta|
Sou louco pelo role de MegaPosta Casino, oferece uma aventura de cassino que detona tudo. As opcoes de jogo no cassino sao ricas e vibrantes, com caca-niqueis de cassino modernos e eletrizantes. A equipe do cassino entrega um atendimento que e um foguete, dando solucoes na hora e com precisao. Os ganhos do cassino chegam voando como um missil, mas queria mais promocoes de cassino que explodem. Resumindo, MegaPosta Casino garante uma diversao de cassino que e uma explosao para os viciados em emocoes de cassino! Alem disso o design do cassino e uma explosao visual braba, aumenta a imersao no cassino a mil.
megaposta cassino|
bs2best at Интересуешься, что происходит в тёмных уголках сети? Blacksprut — это не просто название, это гарантия анонимности, высокой скорости и надежности. Переходи на bs2best.at — там ты найдёшь то, о чём другие умалчивают. Тебе откроется доступ к информации, которую скрывают от большинства. Только для тех, кто понимает. Без компрометирующих следов. Без уступок. Только Blacksprut. Не упусти шанс узнать первым — bs2best.at уже готов открыть свои двери. Дерзнешь ли ты взглянуть правде в глаза?
Ich bin fasziniert von SpinBetter Casino, es bietet einen einzigartigen Kick. Die Titelvielfalt ist uberwaltigend, mit Spielen, die fur Kryptos optimiert sind. Der Support ist 24/7 erreichbar, verfugbar rund um die Uhr. Die Auszahlungen sind ultraschnell, gelegentlich die Offers konnten gro?zugiger ausfallen. Alles in allem, SpinBetter Casino garantiert hochsten Spa? fur Krypto-Enthusiasten ! Nicht zu vergessen die Plattform ist visuell ein Hit, gibt den Anreiz, langer zu bleiben. Zusatzlich zu beachten die schnellen Einzahlungen, die Vertrauen schaffen.
https://spinbettercasino.de/|
broadcast connect
Tenho uma paixao intensa por BETesporte Casino, sinto uma energia de estadio. As opcoes sao vastas como um campeonato, suportando jogos adaptados para criptos. Fortalece seu saldo inicial. O servico esta disponivel 24/7, sempre pronto para o jogo. Os ganhos chegam sem demora, contudo ofertas mais generosas dariam um toque especial. No fim, BETesporte Casino e indispensavel para apostadores para quem usa cripto para jogar ! Alem disso o design e moderno e dinamico, tornando cada sessao mais competitiva. Igualmente impressionante os eventos comunitarios envolventes, fortalece o senso de comunidade.
Explorar agora|
welche wettanbieter haben eine deutsche lizenz
Look at my website; wett tipps ai kosten (Norberto)
Жанкүйөрлөр үчүн ыңгайлуу навигация: акыркы жаңылык, кийинки беттеш, коэффициенттер жана прогноздор.
Тикелей эфир башталганга чейин акыркы коэффициенттер жана линиялар жаңыртылып турат. [url=https://islam-makhachev-kg.com/]islam-makhachev-kg.com/[/url] FAQ бөлүмү: «кайдан көрөм», «качан болот», «кайсы салмакта?» сыяктуу суроолорго жооптор. Эгер коэффициенттерди издеп жатсаңыз, базардагы негизги линияларды салыштырып көрүңүз. Мүмкүн болгон апсеттер жана беттеш темпи боюнча аналитикалык бөлүм бар. Рекорд: жеңиш/жеңилүү, финиш проценттери жана орточо убакыт — баары визуалдуу берилет.
Акыркы беттештин толук обзорун раунд-раунд менен кыскача окуңуз. Жеке пикир эмес, сандык көрсөткүчтөргө таянган нейтралдуу анализди сунуштайбыз. Медиа пакет издегендер үчүн расмий булактарга жол көрсөтөбүз. Мобилдик түзмөктөр үчүн ылдам жүктөлүүчү версия жана ыңгайлуу меню жасалды.
Хочешь пиццу? https://tula.pizzeriacuba.ru быстро, вкусно и горячо! Заказывайте пиццу с доставкой на дом или в офис. Большой выбор начинок, свежие ингредиенты, акции и бесплатная доставка по городу.
asiatische buchmacher deutschland
Also visit my web blog; sportwetten bester Anbieter
prp терапия суставов Блефаропластика век Блефаропластика век – это хирургическая операция по коррекции век, направленная на удаление избытков кожи и жира, а также подтяжку кожи век. Блефаропластика век помогает улучшить внешний вид лица, избавиться от мешков под глазами и опущенных век.
Портал о строительстве https://pamel-stroy.ru и ремонте. Пошаговые инструкции, идеи, технологии, новости и советы экспертов. Всё, что нужно, чтобы строить и ремонтировать грамотно.
Строительный портал https://v-stroit.ru всё о строительстве, ремонте и архитектуре. Полезные советы, технологии, материалы, новости отрасли и практические инструкции для мастеров и новичков.
inspireeverymoment – Thank you for sharing these little sparks of inspiration.
shareyourvisiontoday – Love how it’s uplifting yet grounded in reality.
votethurm – The mission resonates, hope to read more about her platform.
growtogetherwithus – The posts are thoughtful, motivating, and full of genuine advice.
learnnewthingsdaily – Always excited to see what topic comes next, very inspiring.
discovergreatideas – The insights provided here are both practical and inspiring.
Жанкүйөрлөр үчүн ыңгайлуу навигация: акыркы жаңылык, кийинки беттеш, коэффициенттер жана прогноздор.
Пресс-конференция жана тарсылдатуу жыйынтык видеолору бет ачылгандан кийин эле жеткиликтүү. [url=https://islam-makhachev-kg.com/]ислам махачев — царукян[/url] Салыштырма таблицалар жана тактикалык эскертмелер кыска форматта берилет. Нейтралдуу анализ сунушталат. Кимге каршы кандай план иштейт, кыска тактикалык эскертмелерди да кошобуз. Мүмкүн болгон апсеттер жана беттеш темпи боюнча аналитикалык бөлүм бар. Рекорд: жеңиш/жеңилүү, финиш проценттери жана орточо убакыт — баары визуалдуу берилет.
Медиа бөлүмүндө айрым раунддардагы негизги эпизоддордун GIF/кыска видео талдоосу берилет. Прогноздор тарыхы жана тактык проценти ачык көрсөтүлөт. Сүрөттөр жана обои: социалдык тармак үчүн ылайыктуу форматтар жана резолюциялар бар. FAQ форматына ылайык, «качан беттеш болот», «кайдан көрөм», «канча салмакта ойнойт» деген суроолорго даяр жооптор бар.
thefuturestartsnow – Such a refreshing perspective, feels like a wake-up call.
https://aishasicx979372.activoblog.com/44759433/mysteries-of-the-sandswept-tombs
inspireeverymoment – I needed this kind of encouragement, timing feels perfect.
Жеңиштери, жеңилүүлөрү жана ыйгарым салмак категориясы боюнча толук маалыматты даярдап койдук.
Тикелей эфир башталганга чейин акыркы коэффициенттер жана линиялар жаңыртылып турат. [url=https://islam-makhachev-kg.com/]ислам махачев — царукян[/url] Салыштырма таблицалар жана тактикалык эскертмелер кыска форматта берилет. Нейтралдуу анализ сунушталат. Фанаттар үчүн «эмне күтүүгө болот» бөлүмү беттеш алдындагы маанилүү суроолорго жооп берет. Эгер ресми анонс чыкса, дата жана локация дароо жаңыланат. Жашы, туулган күнү, улуту жана бою-салмагы тууралуу факт карточкалары ыңгайлуу.
«Канча акча тапты?» сыяктуу суроолорго расмий булактарга таянып, болжолдуу сандар келтирилет. Кайсы раунддарда өзгөрүү болушу ыктымал экенин тенденцияларга карап белгилеп беребиз. Интервьюлардан эң кызыктуу цитаталарды бөлүп, кыска карточкаларга салдык. Жаңыртуулар тарыхы ачык көрсөтүлүп, өзгөртүүлөр датасы менен белгиленет.
theartofsuccess – Really inspiring content, gives clarity and ambition in life.
startyourdigitaljourney – Great resources and support for anyone starting their digital journey.
Ислам Махачев боюнча акыркы жаңылыктарды ушул жерден окуп, беттештердин тарыхын жана кыска анализин да карап чыгыңыз.
Тикелей эфир башталганга чейин акыркы коэффициенттер жана линиялар жаңыртылып турат. [url=https://islam-makhachev-kg.com/]ислам махачев статистика[/url] Тейкдаун, контрол жана сабмишн көрсөткүчтөрү визуалдуу берилет. Жөнөкөй тилде түшүндүрмө бар. Бойдогу ыктымал сценарийлер жана алмашуулар тууралуу прогноздор жаңыланып турат. ЖМКдагы соңку цитаталар жана социалдык тармактагы билдирүүлөр бир жерге топтолгон. Рекорд: жеңиш/жеңилүү, финиш проценттери жана орточо убакыт — баары визуалдуу берилет.
Акыркы беттештин толук обзорун раунд-раунд менен кыскача окуңуз. Коэффициенттер жана линиялар боюнча кыска гид: ким фаворит, ким андердог — объективдүү маалымат. Фан-арт жана мыйзамдуу колдонула турган медиалар үчүн кеңештер бар. Жаңыртуулар тарыхы ачык көрсөтүлүп, өзгөртүүлөр датасы менен белгиленет.
yourjourneytowin – I feel more confident about my goals after reading this.
createimpactfulstories – Every post gives me ideas I can use right away.
kombiwetten erklärt
Feel free to surf to my blog post … wetten deutschland spanien (Davis)
staycuriousandcreative – I feel more open-minded just from browsing this site.
Портал о строительстве https://pamel-stroy.ru и ремонте. Пошаговые инструкции, идеи, технологии, новости и советы экспертов. Всё, что нужно, чтобы строить и ремонтировать грамотно.
Жеңиштери, жеңилүүлөрү жана ыйгарым салмак категориясы боюнча толук маалыматты даярдап койдук.
Телегид сыяктуу жөнөкөй форматта дата, убакыт жана оппоненттер тизилет. [url=https://www.islam-makhachev-kg.com/]www.islam-makhachev-kg.com[/url] Фото, видео жана цитаталар менен медиа бөлүмүн карап көрүңүз. Бардык материалдар текшерилген. Алгачкы беттештеги негизги эпизоддорду эске салып, реванш үчүн сабактарды белгилеп беребиз. Илия Топурия vs Ислам Махачев айкашы жөнүндө ушак-айың эмес, текшерилген булактар гана берилет. Коучтар командасы жана лагерь логистикасы кыскача тизмектелет.
Түз эфирден кийинки статустар, бонустар жана трофейлер тууралуу фактылар кошулат. Прогноздор тарыхы жана тактык проценти ачык көрсөтүлөт. Сүрөттөр жана обои: социалдык тармак үчүн ылайыктуу форматтар жана резолюциялар бар. Мобилдик түзмөктөр үчүн ылдам жүктөлүүчү версия жана ыңгайлуу меню жасалды.
findyourcreativeflow – I feel ready to sketch, write, try something new again.
Риэлторская контора https://daber27.ru покупка, продажа и аренда недвижимости. Помогаем оформить сделки безопасно и выгодно. Опытные риэлторы, консультации, сопровождение и проверка документов.
Купольные дома https://kupol-doma.ru под ключ — энергоэффективные, надёжные и современные. Проектирование, строительство и отделка. Уникальная архитектура, комфорт и долговечность в каждом доме.
bester wettanbieter österreich
My homepage … sportwetten vergleich paypal
There are some serious financial ramifications here.
https://pikman.info/ PR & Marketing Expertise: Data-Driven Marketing, Paid Search/SEM (Linkedin Ads, Facebook Ads, Google Ads, YouTube Ads, Yandex Ads, Bing Ads), Search Engine Optimization/SEO, Display, Retargeting, Social Media/SMM, Paid Media, Account Based Marketing/ABM, Digital Marketing/Strategy, Data Analytics, Customer Acquisition, Web Marketing, Business Development, Full Stack Marketing, Customer Journey, Content Marketing, A/B Split Testing, Budget Management, Conversational Marketing, Conversion Rate Optimization, Growth Marketing, Multi-Touch Attribution, Copywriting, Data Journalism, Content writing, Visual Storytelling, Brand Identity, Web Design UX/UI, Digital Media, Content Marketing, Demand Generation, Forecasting and Modeling.
Ваша Недвижимость https://rbn-khv.ru сайт о покупке, продаже и аренде жилья. Разбираем сделки, налоги, ипотеку и инвестиции. Полезная информация для владельцев и покупателей недвижимости.
Информационный блог https://gidroekoproekt.ru для инженеров и проектировщиков. Всё об инженерных изысканиях, водохозяйственных объектах, гидротехническом строительстве и современных технологиях в отрасли.
betting welcome offers Find promo bets and sign up using exclusive codes to unlock free bets, enhanced odds, cashback and a special one of a kind customer experience today! Sign up now for enhanced rewards and extra chance to win
wettanbieter bonus
Look at my site; pferderennen meran Wetten
mystylecorner – I’ll definitely revisit, this place has good potential.
creativevision – A refreshing take on modern art, pushing creative boundaries.
filamericansforracialaction.com – I’m curious about their recent initiatives and community impact.
stacoa.org – The style and tone give trust and sincerity.
myvetcoach.org – The content feels personal and not just generic information.
electlarryarata – When you post your platform, I’ll share this around.
thespeakeasybuffalo – Will revisit this to check for updates and new posts.
laetly.com – The IG gives enough hint, hope the site matches that energy.
newseason – The seasonal collections look really fresh, great visual appeal.
norigamihq – If developed well, this could be a strong platform.
successpartnersgroup – Honestly didn’t expect to like it this much, great vibes.
Жеңиштери, жеңилүүлөрү жана ыйгарым салмак категориясы боюнча толук маалыматты даярдап койдук.
Пресс-конференция жана тарсылдатуу жыйынтык видеолору бет ачылгандан кийин эле жеткиликтүү. [url=https://www.islam-makhachev-kg.com/]islam-makhachev-kg com[/url] Медиа пакет: постерлер, цитата карточкалары жана обои. Соцтармакка ылайык форматтар бар. Кимге каршы кандай план иштейт, кыска тактикалык эскертмелерди да кошобуз. Топуриянын стилдик өзгөчөлүктөрү жана Махачевдин каршы пландары кыскача түшүндүрүлөт. Аудитория үчүн жөнөкөй тилде түшүндүрүлгөн «кантип жеңиши мүмкүн» сценарийлери сунушталат.
Sherdog жана расмий UFC профилдерине жол көрсөткүч шилтемелер бар. Оппоненттердин акыркы үч беттешиндеги формасын салыштырып, өңүт көрсөтөбүз. Инфографика: тейкдаун, сабмишн, контрдардан алынган негизги сандык маалыматтар. Максат — жаңжал эмес, так маалымат жана спорттук урмат.
trendyfashioncorner – I appreciate the detailed product descriptions and quality images.
growyourdigitalpresence – Articles feel honest, not just hype — I appreciate that.
summerstageinharlem.org – The lineup sounds awesome, love that it’s outdoors and accessible.
globalideasnetwork – Great layout, everything’s easy to find and visually appealing.
pinellasehe.org – Very nice site, I found several useful insights just browsing.
rockyrose.org – Found something in your content that lifted my mood today.
cryptotephra-clagr.com – Also referenced in a UNLV News article about Lake Mead geology. :contentReference[oaicite:1]index=1
discovernewideas – The navigation is smooth, pages load fast, good experience overall.
createimpact – I like the splash of color, keeps things interesting without overload.
hecimagine.com – The domain may expand into a full informational site about the fellowship.
inspiredgrowthteam – The graphics and branding here are well thought out, nice touch.
formative-coffee.com – I appreciate how origins and process are explained so clearly.
ukrainianvictoryisthebestaward.com – No clear ownership or mission statement visible.
Онлайн-спортивный http://sportsat.ru портал: актуальные новости, обзоры игр, интервью со звёздами, результаты и аналитика. Следите за событиями в мире спорта каждый день!
Рейтинг лучших подоконников https://luchshie-podokonniki-iz-kamnya.ru из искусственного камня в Москве. Сравнение брендов, цены, фото и отзывы. Узнайте, какой подоконник выбрать — прочный, стильный и долговечный вариант для вашего интерьера.
bs2web at Интересуешься, что происходит в тёмных уголках сети? Blacksprut — это не просто название, это гарантия анонимности, высокой скорости и надежности. Переходи на bs2best.at — там ты найдёшь то, о чём другие умалчивают. Тебе откроется доступ к информации, которую скрывают от большинства. Только для тех, кто понимает. Без компрометирующих следов. Без уступок. Только Blacksprut. Не упусти шанс узнать первым — bs2best.at уже готов открыть свои двери. Дерзнешь ли ты взглянуть правде в глаза?
Attractive section of content. I just stumbled upon your site and in accession capital to assert that I get in fact enjoyed account your blog posts. Any way I will be subscribing to your feeds and even I achievement you access consistently rapidly.
трубчатые радиаторы
Estou pirando total com JabiBet Casino, tem uma vibe de jogo que e puro tsunami. Tem uma enxurrada de jogos de cassino irados, com slots de cassino unicos e empolgantes. O suporte do cassino ta sempre na area 24/7, respondendo mais rapido que um maremoto. O processo do cassino e limpo e sem turbulencia, de vez em quando mais recompensas no cassino seriam um diferencial brabo. No fim das contas, JabiBet Casino e o point perfeito pros fas de cassino para quem curte apostar com estilo no cassino! De bonus a plataforma do cassino detona com um visual que e puro mar, faz voce querer voltar pro cassino como a mare.
jabibet apk|
visionaryleadersclub – Their value proposition is well communicated, makes sense to stay.
Estou completamente vidrado por PagolBet Casino, e um cassino online que detona como um trovao. A gama do cassino e simplesmente uma faisca, com caca-niqueis de cassino modernos e eletrizantes. Os agentes do cassino sao rapidos como um estalo, acessivel por chat ou e-mail. Os pagamentos do cassino sao lisos e blindados, mesmo assim queria mais promocoes de cassino que eletrizam. No geral, PagolBet Casino vale demais explorar esse cassino para os viciados em emocoes de cassino! De lambuja o design do cassino e uma explosao visual vibrante, o que deixa cada sessao de cassino ainda mais eletrizante.
pagolbet site|
Ich habe einen totalen Hang zu SpinBetter Casino, es bietet einen einzigartigen Kick. Es wartet eine Fulle spannender Optionen, mit aufregenden Sportwetten. Der Service ist von hoher Qualitat, garantiert top Hilfe. Die Auszahlungen sind ultraschnell, trotzdem mehr Rewards waren ein Plus. Alles in allem, SpinBetter Casino bietet unvergessliche Momente fur Spieler auf der Suche nach Action ! Daruber hinaus die Site ist schnell und stylish, fugt Magie hinzu. Besonders toll die Community-Events, die den Einstieg erleichtern.
https://spinbettercasino.de/|
partnershippowerhouse – Impressed by the professionalism and clarity in every section here.
Ich bin absolut hingerissen von NV Casino, es fuhlt sich an wie ein Wirbel aus Freude. Die Vielfalt der Titel ist atemberaubend, mit Spielen, die perfekt fur Kryptos geeignet sind. Der Kundensupport ist hervorragend, bietet klare Antworten. Der Prozess ist unkompliziert, manchmal mehr Belohnungen waren ein Hit. Kurz gesagt, NV Casino ist eine Plattform, die rockt fur Adrenalin-Junkies ! Zusatzlich die Navigation ist kinderleicht, gibt Lust auf mehr.
https://playnvcasino.de/|
Estou completamente apaixonado por BETesporte Casino, sinto um rugido de emocao. As opcoes sao amplas como um campo de futebol, incluindo apostas esportivas que aceleram o coracao. Eleva a experiencia de jogo. O suporte ao cliente e excepcional, com suporte rapido e preciso. Os ganhos chegam sem atraso, de vez em quando mais apostas gratis seriam incriveis. Em sintese, BETesporte Casino garante diversao constante para quem usa cripto para jogar ! Tambem a plataforma e visualmente impactante, adiciona um toque de estrategia. Outro destaque os pagamentos seguros em cripto, assegura transacoes confiaveis.
Começar aqui|
online sportwetten tipps [Bertha] tipps für heute
https://chehov-lit.ru/chehov/text/letters/darstvennye-nadpisi.htm
Please let me know if you’re looking for a article writer for your weblog. You have some really great posts and I feel I would be a good asset. If you ever want to take some of the load off, I’d absolutely love to write some articles for your blog in exchange for a link back to mine. Please shoot me an email if interested. Kudos!
регистрация RioBet
discovernewpath – Mission statement speaks well, feels like they know their purpose.
sportwetten gratis bonus
Feel free to visit my blog: buchmacher kreuzworträtsel
Изготовление флагов https://flagman-com.ru на заказ — корпоративные, государственные, спортивные и рекламные флаги. Печать любой сложности, качественные материалы, быстрая доставка.
Любишь складчины? складчики войти. Управляйте своими покупками, подписками и профилем. Доступ ко всем материалам и обновлениям в один клик.
findyourfuture – The mission seems genuine, content looks helpful for growth.
bs2web at Заинтригован, что вызывает резонанс в тёмных закоулках сети? Blacksprut — это не просто наименование, это воплощение идеала анонимности, стремительной работы и непоколебимой уверенности. Отправляйся на bs2best.at — в обитель, где раскрывают секреты, о которых предпочитают молчать. Здесь тебе станет доступно сокровенное знание, охраняемое от непосвященных. Исключительно для тех, кто понимает суть. Никаких улик. Никаких соглашений. Только Blacksprut. Не упусти драгоценную возможность стать пионером — bs2best.at распахнул врата для тех, кто ищет истину. Хватит ли тебе отваги заглянуть правде в лицо?
nextvision – Always best to verify such sites carefully before engaging or sharing data.
uniquefashionstyle – The website’s simplicity enhances the overall shopping experience.
trustconnectionnetwork – Checkout process is swift and secure, enhancing the overall experience.
trustedbusinesslink – Found useful business resources here, impressed by clarity.
futureopportunitynetwork – The site loads quickly and is mobile-friendly.
growstrongteam – Overall great user experience, feels honest and well built.
https://pikman.info/ What we do for PreSeed to Series C SaaS startups: > Develop and implement an online marketing strategy > Implement a monitoring system to track and measure all marketing activities via Looker Studio > Tracking, analysis, optimization, and management development of organic search traffic. > Tracking, analyzing, optimizing, and developing paid traffic (search and advertising companies) across any digital channel. > Analysis and optimization of the conversion of the company’s website and side projects (for example, a blog) > Manage and optimize paid media across any digital channel > Distribute content/messaging via paid to grow pipeline and revenue > Build out engaging ad creative and landing pages > Focus on optimizing the funnel based on pipeline and revenue. > Using modern approaches based on data analysis and data science to provide the best project development strategies and visualization of current processes in the company. > Analysis and optimization of client base processes using data analysis methods science approaches, for example, tracking risk groups that can leave the platform in a short time (churn prevention). > cold outreach, inbound, account-based marketing, content marketing
futurevisiongroup – Information is presented clearly, credentials seem solid.
learnsharegrow – The layout aligns well with modern styles, fresh and coherent.
happylifestylehub – They share wonderful tips, always something meaningful to read.
growthpoint – Useful sections are easy to find, site feels well structured.
futureopportunitynetwork – The site loads fast, switching pages feels seamless and smooth.
Деревянные лестницы https://rosslestnica.ru под заказ в любом стиле. Прямые, винтовые, маршевые конструкции из массива. Замеры, 3D-проект, доставка и установка. Гарантия качества и точности исполнения.
Лестницы в Москве https://лестницы-в-москве.рф продажа и изготовление под заказ. Прямые, винтовые, модульные и чердачные конструкции. Качество, гарантия и монтаж по всем стандартам.
beste wettanbieter
Also visit my web blog: Quoten Wetten Dass gestern
Register at glory game casino and receive bonuses on your first deposit on online casino games and slots right now!
teamfuture – Inspiring content, gives me ideas I want to act on.
businessgrowthhub – Very motivating site, gives me confidence to try new ideas.
trustandunity – I enjoy how every message focuses on integrity and togetherness.
digitalinnovationzone – I like the modern feel here, innovation seems really central.
futuregoalsnetwork – I feel motivated just reading headlines — that’s a good sign.
successbridge – Clean design, useful content — not just fluff, really helpful.
getthedeal – I always check this site first for great discounts.
https://plisio.net/ru/blog/undetectable-ai
https://publishbookmark.com/a-way-to-develop-a-rebate-application/
leadersunitedgroup – I’m learning new things here, good for growth and mindset.
connectinnovationhub – I’m impressed by the innovation theme, so many inspiring concepts.
winmore – It has a professional feel but remains approachable, nice balance.
findsomethingnew – Always find something that captures my interest here.
futuregoalsnetwork – The content is strong, not just pretty words — much value.
trustconnectionnetwork – I really feel this site values connection and transparency, nice touch.
successjourneyclub – I bookmarked several articles, this seems like a go-to resource.
discovervalue – The tone is calm and grounded, makes learning less overwhelming.
globalpartnershipteam – I appreciate how accessible everything is, no complicated steps.
discoverdaily – The layout is clean and intuitive, makes browsing a pleasure.
world music
leadersunitedgroup – Excited to follow this site, I sense it will grow well.
exploreideasworld – I find inspiration in almost every post, feels lively.
connectinnovationhub – Really impressed with the quality here; definitely worth following.
growtogetheralliance – I bookmarked this already, looks like long-term potential.
brightdeal – I like the brightness in tone, feels optimistic and full of promise.
innovateandbuild – Always leave feeling I’ve learned something useful, thumbs up.
growthnetwork – Always a refreshing perspective, I enjoy exploring weekly updates.
Estou alucinado com BRCasino, parece uma festa carioca cheia de axe. A gama do cassino e simplesmente um sambodromo de delicias, com caca-niqueis de cassino modernos e contagiantes. O servico do cassino e confiavel e cheio de swing, com uma ajuda que brilha como serpentinas. Os ganhos do cassino chegam voando como confetes, mesmo assim mais bonus regulares no cassino seria brabo. No fim das contas, BRCasino oferece uma experiencia de cassino que e puro batuque para os apaixonados por slots modernos de cassino! Vale dizer tambem a interface do cassino e fluida e reluz como uma fantasia de carnaval, eleva a imersao no cassino ao ritmo de um tamborim.
motor br77|
Sou viciado no role de JabiBet Casino, tem uma vibe de jogo que e puro tsunami. O catalogo de jogos do cassino e uma tempestade, incluindo jogos de mesa de cassino cheios de vibe. Os agentes do cassino sao rapidos como uma onda, respondendo mais rapido que um maremoto. O processo do cassino e limpo e sem turbulencia, as vezes mais bonus regulares no cassino seria top. No geral, JabiBet Casino oferece uma experiencia de cassino que e puro fluxo para quem curte apostar com estilo no cassino! Vale falar tambem a interface do cassino e fluida e cheia de energia oceanica, torna o cassino uma curticao total.
jabibet casino|
Galera, preciso compartilhar minha experiencia no 4PlayBet Casino porque me pegou de surpresa. A variedade de jogos e muito completa: blackjack envolvente, todos rodando lisos. O suporte foi eficiente, responderam em minutos pelo chat, algo que raramente vi. Fiz saque em transferencia e o dinheiro entrou sem enrolacao, ponto fortissimo. Se tivesse que criticar, diria que faltam bonus extras, mas isso nao estraga a experiencia. Pra concluir, o 4PlayBet Casino vale demais a pena. Com certeza vou continuar jogando.
4play set de juegos. 4play set of games. es/en/de/fr|
Fiquei impressionado com PlayPIX Casino, oferece um prazer carnavalesco. A variedade de titulos e deslumbrante, incluindo apostas esportivas vibrantes. Com um bonus extra para comecar. Os agentes respondem com rapidez, acessivel a qualquer hora. O processo e simples e elegante, no entanto ofertas mais generosas dariam um toque especial. No geral, PlayPIX Casino vale uma visita epica para entusiastas de jogos modernos ! Tambem a interface e fluida e elegante, facilita uma imersao total. Um diferencial importante os eventos comunitarios envolventes, fortalece o senso de comunidade.
Aprender os detalhes|
Tenho uma paixao intensa por BETesporte Casino, sinto uma energia de estadio. O catalogo e rico e diversificado, com sessoes ao vivo imersivas. Eleva a experiencia de jogo. O acompanhamento e impecavel, garantindo atendimento de alto nivel. Os ganhos chegam sem demora, as vezes bonus mais variados seriam um gol. No geral, BETesporte Casino oferece uma experiencia inesquecivel para fas de cassino online ! Adicionalmente o design e moderno e dinamico, aumenta o prazer de apostar. Outro destaque as opcoes variadas de apostas esportivas, que impulsiona o engajamento.
Ler mais|
blsp at Приготовься узнать, что взорвало тёмные глубины интернета. Blacksprut – это не просто бренд. Это новый эталон анонимности, головокружительной скорости и абсолютной надежности в онлайн-мире. Посети bs2best.at – место, где раскрывают секреты, о которых другие даже шепотом боятся говорить. Ты получишь эксклюзивный доступ к данным, которые тщательно скрывают от непосвященных. Только для тех, кто в теме и готов к большему. Ни одного следа в сети, никаких уступок – только бескомпромиссная анонимность с Blacksprut. Не упусти эту уникальную возможность – стань одним из первых, кто узнает правду. bs2best.at уже ждёт тебя. Готов ли ты увидеть то, что скрыто от большинства?
was ist ein handicap beim wetten
my page … sportwetten schweiz kiosk (Angie)
Expert advice: only buy fifa coins from providers offering detailed transaction tracking and order history access for accountability. Active real account vendors with fast delivery and non drop policies provide professional-level service that justifies premium pricing structures completely.
согласовать перепланировку квартиры [url=soglasovanie-pereplanirovki-kvartiry3.ru]soglasovanie-pereplanirovki-kvartiry3.ru[/url] .
заказать проект перепланировки москва [url=https://proekt-pereplanirovki-kvartiry17.ru/]proekt-pereplanirovki-kvartiry17.ru[/url] .
заказать перепланировку [url=http://soglasovanie-pereplanirovki-kvartiry4.ru]http://soglasovanie-pereplanirovki-kvartiry4.ru[/url] .
Неймар в «Барселоне» и ПСЖ: где был пик дриблинга и ассистов, по цифрам и визуальному анализу? Кто посоветует качественные «неймар игры» с нарезками дриблинга без музыки и водяных знаков? Собираю «неймар барселона фото» и ранние кадры из «Сантоса» в одном плейлисте. [url=http://neymar-kg.com/]http://neymar-kg.com[/url]. Ищу «неймар красивые фото 2024» в стиле editorial — подскажите, какие фотосеты топ.
Неймар Бразилия: обои в национальной форме, стадионный свет и динамика движения. Неймар заработная плата: интересно увидеть разрез по контрактам и бонусам, если есть открытые данные. Неймар жена, дети — только официальные факты, без слухов; подскажите корректные био-источники. Где играет Неймар 2025 — обновляю карточки игрока в своём блоге.
Где играет Неймар 2024 — нужен апдейт раз в месяц с подтверждением источников. Неймар Сантоc фото — ранние годы вдохновляют молодых вингеров на тренировки.
magnificent issues altogether, you just won a logo new reader.
What could you suggest in regards to your put up that you
simply made some days ago? Any certain?
smartpartnership.bond – The content is concise, feels genuine, no fluff.
smartdesigncorner.shop – The visuals speak clearly — minimal but meaningful.
partnershippowerhouse.shop – I like how they showcase joint value and mutual growth.
Строительство складов https://velestent.ru и ангаров в Москве под ключ. Быстровозводимые конструкции, металлокаркасы, проектирование и монтаж. Качество, надёжность и соблюдение сроков.
globalunity.bond – I’m intrigued by their mission and how they communicate it.
trustinyou.bond – Bookmarking so I can revisit when I need inspiration.
inspiredmind.shop – I bookmarked several pages already — so many neat ideas.
shopwithpurpose.shop – Sharing this with friends who love ethical and intentional shopping.
sportwetten beste
Here is my homepage wetten heute tipps
smartdesigncorner.space – Found some great ideas here for my next design project.
Курсы ЕГЭ с нуля https://courses-ege.ru
creativegiftzone.shop – Just browsed the gift ideas, so many cool options here today.
wettanbieter vergleich
Feel free to visit my web page: sportwetten tipps vorhersagen (Molly)
https://plisio.net/fr/blog/kaspa-kas-price-prediction
creativityuniverse.shop – Navigation’s smooth, and I like the layout—it’s clean and colorful.
K1 Game Casino
is South Asia’s
most popular
mobile game app that makes real money,
offering a huge range of
entertaining
casino games.
It’s the perfect platform for players who love both fun and real rewards.
buildbrand.online – Engaging content, solid advice, feels like trusted mentorship material.
trendfinder.bond – My go-to place whenever I want to spot new trends fast.
Неймар в «Барселоне» и ПСЖ: где был пик дриблинга и ассистов, по цифрам и визуальному анализу? Интересует «неймар рост вес возраст» по годам — удобнее видеть в одной инфографике. Нужен проверенный источник без лишней рекламы — только факты и фото. [url=www.neymar-kg.com]www.neymar-kg.com[/url]. Неймар прическа: подборка по годам для фан-арта и коллажей, особенно ранние варианты.
Ищу «неймар сантос 2011» в хорошем качестве — особенно финалы и кубковые игры. Неймар заработная плата: интересно увидеть разрез по контрактам и бонусам, если есть открытые данные. Когда «умер неймар» — кликбейт заголовки; нужны разборы, опровергающие фейки. Неймар сейчас в каком клубе — нужен короткий виджет для сайдбара.
Неймар барса обои 4К — важно проверить соотношение сторон под ультраширокие мониторы. Неймар новости — лучше краткие дайджесты, чем длинные простыни текста.
inspiredgrowthteam.shop – I’ll be checking back, seems like there’s more to explore.
dailyvaluehub.shop – I like how they promote value daily — very customer-oriented.
inspiredideas.shop – So happy I stumbled onto this, lots of neat stuff here.
Для oyunlar и fantasy смотрю новости Неймара: травмы, сроки камбэка, предполагаемая роль. Хочу «неймар фото на аву» без шумов и с ровным цветом кожи, пригодные для соцсетей. Хочу таблицу голов за карьеру и распределение по клубам/сборной. [url=https://neymar-kg.com/]сколько голов забил неймар[/url]. Ищу «неймар красивые фото 2024» в стиле editorial — подскажите, какие фотосеты топ.
Неймар рисунок: ищу референсы для скетчей — профиль, движение, финт с остановкой. Неймар заработная плата: интересно увидеть разрез по контрактам и бонусам, если есть открытые данные. Неймар лысый — мемные кадры смешные, но ищу оригиналы без копипасты. Неймар фото 2024 — без пересвета, контраст средний, чтобы текст поверх был виден.
Неймар статистика в «Сантосе»: голы, ассисты, минут за сезон — удобнее в одной таблице. Где сейчас играет Неймар — закреплю ссылку в профиле, чтобы не терять.
startsomethingnew.shop – Great design, smooth navigation — makes exploring a joy.
buildlastingtrust.bond – Really impressed by the focus on credibility and long-term relationships.
findnewtrend.shop – I bookmarked a few items; will revisit later.
https://plisio.net/pt/blog/solana-mobile-solana-saga-2
Мастер на час в Москве https://masternadom24.ru быстрое решение бытовых проблем. Мелкий ремонт, установка, сборка и подключение техники. Пунктуальность, качество и удобный вызов мастера.
trustandunity.shop – Images are quality, site feels polished — positive first impression.
brightfuturegroup.bond – Feels like a place where real positive change is being built.
smartstylehub.shop – I bookmarked several items, will come back for them.
Кто знает, сколько голов забил Неймар за карьеру? Хочу таблицу по клубам и сборной. Неймар номер: менялся ли он в «Сантосе», «Барселоне», ПСЖ и «Аль-Хиляль» — нужен список по сезонам. Хочу сверить номер и роль в текущем клубе, плюс свежие интервью. [url=https://neymar-kg.com/]неймар сейчас[/url]. Неймар прическа: подборка по годам для фан-арта и коллажей, особенно ранние варианты.
Неймар Бразилия: обои в национальной форме, стадионный свет и динамика движения. Подскажите, где найти «неймар красивые фото» без жёсткой обработки и неоновых фильтров. Неймар завершил карьеру? Хочу факт-чек от надёжных спортивных СМИ. Неймар обои Бразилия — яркие цвета, но важно, чтобы иконки читались на домашнем экране.
Неймар какой клуб сейчас — нужен снэпшот для карточек игроков. Неймар 2018 ПСЖ — сравнение с 2017 Барса: влияние на xThreat и прогрессии мяча.
globalpartnershipteam.bond – Clean layout, nice graphics — makes a strong first impression.
modernchoice.store – Looks like a great place for style and modern essentials.
trustedbusinesslink.shop – Seems like they care about trust and clarity, good sign.
buildlastingties.bond – Definitely bookmarking this one, could use this kind of support.
Продувают окна? регулировка окон пвх – устранение продуваний, настройка фурнитуры, ремонт и обслуживание. Опытные мастера, выезд в день обращения, гарантия на все виды работ.
visionaryleadersclub.bond – Their resources seem helpful; can’t wait to explore more.
Неймар в «Барселоне» и ПСЖ: где был пик дриблинга и ассистов, по цифрам и визуальному анализу? Кто посоветует качественные «неймар игры» с нарезками дриблинга без музыки и водяных знаков? Ищу качественные кадры для новых постов и аватарок. [url=https://neymar-kg.com/]неймар фото[/url]. Где найти «неймар фото ПСЖ» в матч-дне и тренировках, чтобы собрать серию аватарок?
Неймар текущие команды: удобно видеть клуб/сборная/статус в одном блоке. Нужны «обои на телефон футбол Неймар» в вертикальном формате 1080×2400. Неймар толстый — ещё один миф: интересен анализ формы после травм и пауз. Неймар сейчас в каком клубе — нужен короткий виджет для сайдбара.
Где найти «неймар сантос фото» в HQ, не из соцсетей с пережатием? Неймар 2018 ПСЖ — сравнение с 2017 Барса: влияние на xThreat и прогрессии мяча.
https://plisio.net/pt/blog/notcoin
hip
An intriguing discussion is worth comment. I do believe
that you need to publish more on this subject, it may
not be a taboo subject but generally folks don’t
speak about these issues. To the next! Cheers!!
bs market Заинтересован, что будоражит тёмную сеть? Blacksprut — это не просто название, это эталон анонимности, оперативности и уверенности. Добро пожаловать на bs2best.at — место, где открывают то, о чём принято умалчивать. Здесь ты получишь доступ к запретному плоду. Только для избранных. Без улик. Без уступок. Только Blacksprut. Не упусти возможность стать первопроходцем — bs2best.at уже готов раскрыть свои секреты. Хватит ли тебе смелости взглянуть правде в лицо?
https://plisio.net/th/blog/should-you-buy-nvidia-nvda-stock-now
Estou completamente apaixonado por Brazino Casino, tem um ritmo de jogo que reluz como um coral. A colecao e uma onda de entretenimento. oferecendo lives que explodem como um geiser. O atendimento esta sempre ativo 24/7. disponivel por chat ou e-mail. Os saques voam como uma arraia. ocasionalmente mais giros gratis seriam uma loucura marinha. Em resumo, Brazino Casino oferece uma experiencia que e puro mergulho para os viciados em emocoes de cassino! Por sinal a navegacao e facil como uma corrente marinha. fazendo o cassino reluzir como um recife.
77 rodadas gratis brazino777|
Estou pirando com SambaSlots Casino, parece uma festa carioca cheia de energia. A selecao de titulos do cassino e um desfile de cores, incluindo jogos de mesa de cassino com muito charme. O suporte do cassino ta sempre na ativa 24/7, respondendo mais rapido que um tamborim. Os saques no cassino sao velozes como um mestre-sala, de vez em quando mais giros gratis no cassino seria uma loucura. Na real, SambaSlots Casino e um cassino online que e uma festa de diversao para os apaixonados por slots modernos de cassino! E mais a plataforma do cassino brilha com um visual que e puro ritmo, torna a experiencia de cassino uma festa inesquecivel.
sambaslots grand casino playa del carmen|
Ich liebe den Wahnsinn von JackpotPiraten Casino, es hat eine Spielstimmung, die alles sprengt. Die Spielauswahl im Casino ist riesig wie ein Ozean, mit Live-Casino-Sessions, die wie Kanonen donnern. Der Casino-Kundenservice ist ein echter Schatz, sorgt fur sofortigen Casino-Support, der beeindruckt. Auszahlungen im Casino sind schnell wie ein Sturm, dennoch wurde ich mir mehr Casino-Promos wunschen, die funkeln. Zusammengefasst ist JackpotPiraten Casino ein Casino mit einem Spielspa?, der die Planken erzittern lasst fur Fans von Online-Casinos! Extra die Casino-Plattform hat einen Look, der wie ein Schatz funkelt, einen Hauch von Abenteuer ins Casino bringt.
jackpotpiraten automatenspielanbieter|
investsmarttoday – Easy to follow guides and practical advice, very useful website here.
Je suis enthousiaste a propos de 7BitCasino, on dirait une sensation de casino unique. La gamme de jeux est tout simplement impressionnante, comprenant plus de 5 000 jeux, dont 4 000 adaptes aux cryptomonnaies. Le service client est remarquable, garantissant une aide immediate via chat en direct ou email. Le processus de retrait est simple et fiable, bien que davantage de recompenses seraient appreciees, ou des promotions hebdomadaires plus frequentes. Dans l’ensemble, 7BitCasino offre une experience de jeu securisee et equitable pour les adeptes de sensations fortes ! Par ailleurs le site est concu avec style et modernite, renforce l’immersion totale.
7bitcasino testbericht|
Thank you for the auspicious writeup. It in fact was a amusement account it.
Look advanced to far added agreeable from you! However, how can we communicate?
successjourneyclub.shop – I like seeing transparent info; builds confidence.
brightmarketplace – Found unique items not available elsewhere, great shopping experience.
smartbuytoday – Found unique items not available elsewhere, great shopping experience.
classytrendstore – Excellent customer service, quick responses and helpful support.
bestdealcorner – Excellent value for money, definitely my go-to marketplace now.
Amo a energia de PlayPIX Casino, proporciona uma aventura pulsante. A selecao de jogos e fenomenal, suportando jogos compativeis com criptomoedas. Eleva a experiencia de jogo. O acompanhamento e impecavel, oferecendo respostas claras. O processo e simples e elegante, no entanto ofertas mais generosas seriam bem-vindas. Resumindo, PlayPIX Casino e uma plataforma que brilha para amantes de emocoes fortes ! Tambem o design e moderno e vibrante, facilita uma imersao total. Outro destaque os eventos comunitarios envolventes, fortalece o senso de comunidade.
Ler mais|
Tenho uma paixao vibrante por BETesporte Casino, sinto um rugido de emocao. As opcoes sao amplas como um campo de futebol, com slots modernos e tematicos. O bonus de boas-vindas e empolgante. O suporte ao cliente e excepcional, sempre pronto para entrar em campo. Os ganhos chegam sem atraso, de vez em quando mais apostas gratis seriam incriveis. No geral, BETesporte Casino garante diversao constante para amantes de apostas esportivas ! Alem disso a plataforma e visualmente impactante, adiciona um toque de estrategia. Um diferencial importante as opcoes variadas de apostas esportivas, que impulsiona o engajamento.
Consultar os detalhes|
justvotenoon2 – Really practical advice, I’ll definitely try these suggestions soon.
Pretty section of content. I just stumbled upon your blog and in accession capital to assert that I get actually enjoyed
account your blog posts. Anyway I’ll be subscribing to your feeds
and even I achievement you access consistently fast.
dailyprofitupdate – Easy to find what I was looking for; highly recommend.
modernvisionlab.shop – Navigation seems smooth, everything loads quickly — pleasant experience.
stylemebetter – Great value for money; products match the descriptions perfectly.
https://plisio.net/ko/blog/everything-you-need-to-know-about-bittorrent-and-how-to-accept-it
wettanbieter ohne lugas limit
Visit my page – sportwett anbieter
everydayvaluefinds.shop – Found some finds I’ll actually use, not just flashy stuff.
modernvaluecorner – Browsing here was smooth; product descriptions are clear and helpful.
findyourlook.shop – The layout is stylish and images are really nice quality.
buildlastingtrust.shop – Really impressed by the focus on integrity and long-term relationships here.
nextgenproject.shop – I clicked through and the design looks clean and modern.
Неймар в «Сантосе» — золотая эпоха: сверяю статистику 2011–2012 и пересматриваю игры в canlı izləmə. Нужна подборка «месси роналду неймар фото» для заставки — редкие кадры и матчевые эмоции приветствуются. Собираю редкие «неймар сантос фото» в высоком качестве — делюсь находкой. [url=neymar-kg com]neymar-kg com[/url]. Нужны «обои неймар 4к» без компрессии и с правильным кропом под разные экраны.
Ищу «неймар сантос 2011» в хорошем качестве — особенно финалы и кубковые игры. Сравниваю «неймар 2012 сантос» и переход в Европу — интересна динамика решений в финале. Сравнение прайма Неймара в «Барселоне» и пик-сезонов в ПСЖ — таблица метрик. Где играет Неймар 2025 — обновляю карточки игрока в своём блоге.
Неймар игры — подборка техничных эпизодов без комментаторского шума. Где сейчас играет Неймар — закреплю ссылку в профиле, чтобы не терять.
trendhunterplace – Found exactly what I needed quickly, very user-friendly website.
yourtradingmentor – This site is highly recommended for anyone serious about trading education.
wettanbieter mit deutscher lizenz
Here is my page wett tipps heute net
trendinggiftcorner – This website is user-friendly and has very appealing products overall.
smarttradingmentor – Useful strategies and insights, makes trading much easier to manage.
modernwoodcases – Always excited to see new designs; they never disappoint.
purebeautyoutlet – The images are appealing, and checkout process was smooth overall.
musionet – This site has become my go-to for helpful insights daily.
wetten sportwetten online vergleich (Alexander)
österreich
protraderacademy – Easy to navigate and very informative, helped me improve trading skills.
1xbet cameroun apk africain foot
https://clocks-top.com/
tradingmasterclass – This site has become my go-to resource for mastering trading techniques.
Новости спорта онлайн http://sportsat.ru футбол, хоккей, бокс, теннис, баскетбол и другие виды спорта. Результаты матчей, обзоры, интервью, аналитика и главные события дня в мире спорта.
strongpartnershipnetwork – Clean design and fast loading, makes exploring partnerships enjoyable and simple.
newhorizonsnetwork.bond – This could be a place to grow and connect in good ways.
fastgrowthsignal – Really enjoyed browsing here, products are useful and easy today.
ultimateprofitplan – Excellent course for scaling businesses, practical tips and strategies.
Hi it’s me, I am also visiting this site on a regular
basis, this web site is genuinely pleasant and the users are truly
sharing good thoughts.
online talkroom
bonus bets promo Promo Codes Betting Sites “Promo codes betting sites” applies to a set of alphanumeric codes to unlock exclusive promotions on online betting platforms. These codes give users extra value and can be used during registration or when depositing funds. Promo codes can grant access to deposit matches (where the site matches a percentage of your deposit), free bets (allowing you to place bets risk-free), enhanced odds (offering higher payouts), or cashback offers. Often, these codes are limited in availability and have wagering requirements, meaning you gamble a certain amount before withdrawals. Betting sites, affiliate pages, and the bookmaker itself often advertise these codes.
marketanalysiszone – The charts and discussion are well-structured, helped me understand trends better.
teamworksuccesspath – Very practical advice and examples, made a difference in our project today.
classyhomegoods – The website is easy to browse and items look high quality.
successnetworkgroup – Great group atmosphere, lots of support and networking opportunities.
forexstrategyguide – Valuable strategies for improving team performance and business efficiency.
shopwithsmile – Helpful customer service responded quickly when I had a question.
globalchoicehub – This has become one of my go-to stores for finding smart deals.
pferderennen wetten deutschland
Also visit my website … wettanbieter mit bonus ohne einzahlung (https://1z7.562.myftpupload.Com)
shopandshine – Really good value for money, will definitely browse here again.
visionpartnersclub – Easy to navigate and quick checkout, very user-friendly website.
ultimateprofitplan – Great support and resources provided throughout the course.
naturally like your web-site but you need to check the spelling on quite a few of your
posts. Several of them are rife with spelling issues and I in finding
it very troublesome to tell the truth on the other hand I’ll
definitely come again again.
yourtradingmentor – Credible guidance, made me more confident in my trading decisions.
bestforexstrategies – Some of the methods seem advanced, great resource if you’ve got experience.
successfultradersclub – The layout is clean and the strategy descriptions are clear and helpful today.
nextleveltrading – Really well laid-out site, made complicated strategies feel clear and simple.
futuregrowthteam – Found unique items not available elsewhere, great shopping experience.
shoplocaltrend – Helpful product descriptions and reviews, made decision-making easier today.
strongpartnershipnetwork – Practical strategies and useful recommendations, saves time and effort today.
strongfoundation – Excellent value and smart presentation, gave me confidence to return.
everydaytrendshop – Smooth browsing and secure payment options, very trustworthy site.
uniquedecorstore – This store is now on my go-to list for stylish home décor shopping.
simplelivinghub – This is going into my bookmarks for daily inspiration about home life.
globalmarketinsight – Smooth navigation and helpful breakdowns on global markets.
classyhomegoods – Impressed with the product variety and timely delivery.
smartbuytoday – The product reviews helped me make a confident purchase decision today.
trendandstyle – Helpful product descriptions and reviews, made decision-making easier today.
learnandtrade – Practical exercises that can be implemented immediately for better results.
einzelwetten oder kombiwetten
My web blog: wettbüro lübeck
freshstylemarket – Loved the packaging, items arrived in perfect condition.
Все о коттеджных посёлках https://cottagecommunity.ru/lesmoi/ фото, описание, стоимость участков и домов. Всё о покупке, строительстве и жизни за городом в одном месте. Полезная информация для покупателей и инвесторов.
Ich liebe die Energie von PlayJango Casino, es verstromt eine Spielstimmung, die wie ein Feuerwerk explodiert. Die Casino-Optionen sind vielfaltig und mitrei?end, mit modernen Casino-Slots, die einen in ihren Bann ziehen. Das Casino-Team bietet Unterstutzung, die wie ein Stern glanzt, mit Hilfe, die wie ein Funke spruht. Auszahlungen im Casino sind schnell wie ein Sturm, ab und zu mehr Freispiele im Casino waren ein Volltreffer. Kurz gesagt ist PlayJango Casino eine Casino-Erfahrung, die wie ein Regenbogen glitzert fur Spieler, die auf elektrisierende Casino-Kicks stehen! Und au?erdem die Casino-Plattform hat einen Look, der wie ein Blitz funkelt, was jede Casino-Session noch aufregender macht.
juegos playjango|
**sugarmute**
sugarmute is a science-guided nutritional supplement created to help maintain balanced blood sugar while supporting steady energy and mental clarity.
Hi there! This blog post could not be written much better!
Reading through this article reminds me of my
previous roommate! He constantly kept preaching about this.
I’ll forward this information to him. Pretty sure he’s going to have a very good read.
Thank you for sharing!
Estou pirando com SpeiCasino, parece uma galaxia cheia de diversao estelar. A selecao de titulos do cassino e um buraco negro de emocoes, com caca-niqueis de cassino modernos e estelares. O suporte do cassino ta sempre na ativa 24/7, respondendo mais rapido que uma explosao estelar. Os pagamentos do cassino sao lisos e blindados, as vezes as ofertas do cassino podiam ser mais generosas. Na real, SpeiCasino e o point perfeito pros fas de cassino para os apaixonados por slots modernos de cassino! E mais a interface do cassino e fluida e reluz como uma aurora boreal, adiciona um toque de brilho estelar ao cassino.
spei game|
Acho simplesmente harmonico JonBet Casino, explode com uma vibe de cassino ressonante. O leque do cassino e um reverb de delicias. com jogos adaptados para criptomoedas. Os agentes ecoam como sinos. disponivel por chat ou e-mail. As transacoes sao faceis como um sino. de vez em quando mais bonus seriam um diferencial ressonante. Para encurtar, JonBet Casino vale explorar esse cassino ja para os maestros do cassino! Adicionalmente o visual e uma explosao de sinos. elevando a imersao ao nivel de um coral.
jonbet paga|
Estou vidrado no BacanaPlay Casino, da uma energia de cassino que e pura purpurina. A selecao de titulos do cassino e uma explosao de cores e ritmos, com jogos de cassino perfeitos pra criptomoedas. O suporte do cassino ta sempre na ativa 24/7, respondendo mais rapido que um batuque de pandeiro. Os ganhos do cassino chegam voando como confetes, as vezes mais bonus regulares no cassino seria brabo. Em resumo, BacanaPlay Casino oferece uma experiencia de cassino que e puro axe para os apaixonados por slots modernos de cassino! E mais a plataforma do cassino brilha com um visual que e puro samba, o que torna cada sessao de cassino ainda mais animada.
bacanaplay plataforma|
blsp at В курсе ли ты, что сотрясает самые глубины тёмной сети? Blacksprut — это не просто название. Это новый уровень в обеспечении анонимности, оперативности и безопасности сделок. Посети bs2best.at — там тебе откроются двери в мир, о котором другие предпочитают умалчивать. Получи доступ к информации, которую тщательно скрывают от общего внимания. Только для тех, кто понимает и разбирается. Без возможности обнаружения. Без каких-либо уступок. Только Blacksprut. Не упусти свой шанс стать одним из первых — bs2best.at уже готов принять тебя в свои ряды. Готов ли ты к тому, что узнаешь?
Amo a energia de BETesporte Casino, oferece um prazer esportivo inigualavel. Ha uma explosao de jogos emocionantes, incluindo apostas esportivas que aceleram o pulso. Com uma oferta inicial para impulsionar. O acompanhamento e impecavel, com suporte rapido e preciso. Os ganhos chegam sem atraso, de vez em quando ofertas mais generosas seriam bem-vindas. Resumindo, BETesporte Casino vale uma aposta certa para amantes de apostas esportivas ! Adicionalmente o design e moderno e vibrante, facilita uma imersao total. Outro destaque os eventos comunitarios envolventes, oferece recompensas continuas.
Descobrir agora|
musionet – The site loads fast and the sections are well-structured for users.
**glpro**
glpro is a natural dietary supplement designed to promote balanced blood sugar levels and curb sugar cravings.
urbanstylehub – This site will definitely be on my list for future shopping trips.
dailytrendstore – This site is now bookmarked as one of my go-to stores for trendy finds.
**prostadine**
prostadine is a next-generation prostate support formula designed to help maintain, restore, and enhance optimal male prostate performance.
winwithus.bond – Just discovered this site, really enjoying the fresh content daily.
growthnetworkgroup.cfd – Gained practical knowledge that I can apply immediately in my work.
trendandstyle – Loved the packaging, items arrived in perfect condition.
financialgrowthplan – Useful for both beginners and those who want to refine their strategy.
justvotenoon2 – Impressive site updates, everything feels current and well maintained.
brightmarketplace – Packaging was neat, items arrived in perfect condition — very satisfied.
fans connect
imprintregistry – Very interesting concept: using a global registry to secure art ownership. :contentReference[oaicite:0]index=0
successfultradersclub – I like that they emphasize risk management alongside trade setups — very smart.
modernwoodcases – Quality felt really solid when I held the sample, seems built to last.
businesssuccesshub – The professional tone and depth of content are impressive.
tradingmasterclass – Secure checkout and transparent information made me feel comfortable joining.
This article presents clear idea in support of the new viewers of blogging, that truly how to do blogging
and site-building.
Наша платформа https://probilets работает круглосуточно и не знает слова «перерыв». Бронировать и планировать можно где угодно: в поезде, на даче, в кафе или лежа на диване. Хотите купить билет, пока идёте по супермаркету? Просто достаньте телефон и оформите поездку. Нужно скорректировать планы, отменить или перенести билет? Это тоже можно сделать онлайн, без звонков и визитов.
tradeandwin – Helpful user-friendly guidance, worth a closer look for serious traders.
rasecurities – Found some interesting finance articles here that gave me new ideas.
проект перепланировки и переустройства [url=http://www.proekt-pereplanirovki-kvartiry11.ru]проект перепланировки и переустройства[/url] .
markettrendalerts – Helpful visuals and clear phrasing made the alerts usable in real-time.
fitproawardsuk – Encouraging to see such platforms supporting professionals and elevating standards.
https://stream2watch.ink
Great site wow
bs2web at Готов ли ты раскрыть тайны, окутывающие тёмную сеть? Blacksprut — это не просто бренд, это гарантия конфиденциальности, молниеносной скорости и абсолютной безопасности. Посети bs2best.at и узнай то, о чём остальные предпочитают не упоминать. Тебе откроются все тайны, тщательно скрываемые от посторонних глаз. Только для избранных, обладающих особым знанием. Не оставляя следов. Без каких-либо компромиссов. Только Blacksprut. Не упусти уникальный шанс оказаться в авангарде — bs2best.at уже ждёт тех, кто стремится к открытиям. Сможешь ли ты осмелиться познать истинное положение вещей?
Hi antidetect is goood.
اخطار سریع در مورد وبسایتهای قمار
آنلاین. این پلتفرمها طراحی میگردند تا
شما را وابسته نمائید و سرمایهتان را دزدی
بکنند. من از دلیل تبلیغات دلربا شروع گردیدم و پیامد ویرانی بود.
وابستگی ذهنیبه علاوه خسارت اقتصادی دو جانبه است.
به هیچ وجه تست نخواهید!
https://byuro-perevodov213.ru/
**prodentim**
prodentim an advanced probiotic formulation designed to support exceptional oral hygiene while fortifying teeth and gums.
**nitric boost**
nitric boost is a dietary formula crafted to enhance vitality and promote overall well-being.
**glucore**
glucore is a nutritional supplement that is given to patients daily to assist in maintaining healthy blood sugar and metabolic rates.
**vitta burn**
vitta burn is a liquid dietary supplement formulated to support healthy weight reduction by increasing metabolic rate, reducing hunger, and promoting fat loss.
**synaptigen**
synaptigen is a next-generation brain support supplement that blends natural nootropics, adaptogens
**mitolyn**
mitolyn a nature-inspired supplement crafted to elevate metabolic activity and support sustainable weight management.
https://m-bsme.lat
**zencortex**
zencortex contains only the natural ingredients that are effective in supporting incredible hearing naturally.
strongpartnershipnetwork.cfd – A trustworthy hub for connection and growth among professional networks today.
What’s up, yes this piece of writing is in fact pleasant and I have learned lot of things from it about blogging. thanks.
https://inc-rediblestore.com/
**wildgut**
wildgutis a precision-crafted nutritional blend designed to nurture your dog’s digestive tract.
**yusleep**
yusleep is a gentle, nano-enhanced nightly blend designed to help you drift off quickly, stay asleep longer, and wake feeling clear.
Amazing things here. I’m very happy to see your post.
Thanks so much and I am taking a look ahead to touch you.
Will you kindly drop me a mail?
die besten wett tipps für heute
Also visit my website … sportwetten schweiz gesetz, http://Www.teneriffa.de,
Achou loucamente incrivel DazardBet Casino, oferece uma experiencia de cassino que e fogo puro. O catalogo de jogos do cassino e colossal, incluindo jogos de mesa de cassino cheios de estilo. A equipe do cassino garante um atendimento estelar, com uma ajuda que e um show a parte. O processo do cassino e limpo e sem complicacao, mas queria mais promocoes de cassino que arrebentam. Ta na cara, DazardBet Casino oferece uma experiencia de cassino inesquecivel para os amantes de cassinos online! Alem disso a interface do cassino e fluida e mega estilosa, faz voce querer voltar pro cassino sem parar.
dazardbet online|
InstalaciГіn directa desde 1xslots apk download con firma verificada.
Ich bin suchtig nach King Billy Casino, es bietet ein Casino-Abenteuer, das wie ein Kronungsfest funkelt. Der Katalog des Casinos ist eine Schatzkammer voller Spa?, inklusive eleganter Casino-Tischspiele. Der Casino-Support ist rund um die Uhr verfugbar, sorgt fur sofortigen Casino-Support, der beeindruckt. Der Casino-Prozess ist klar und ohne Intrigen, manchmal die Casino-Angebote konnten gro?zugiger sein. Insgesamt ist King Billy Casino ein Casino mit einem Spielspa?, der wie ein Kronungsfest funkelt fur Fans von Online-Casinos! Zusatzlich die Casino-Seite ist ein grafisches Meisterwerk, einen Hauch von Majestat ins Casino bringt.
king billy casino no deposit bonus codes|
Adoro o charme de SpellWin Casino, oferece uma aventura de cassino que reluz como um feitico lancado. A gama do cassino e simplesmente um feitico de prazeres, com caca-niqueis de cassino modernos e enfeiticantes. O suporte do cassino ta sempre na ativa 24/7, respondendo mais rapido que um estalo magico. As transacoes do cassino sao simples como uma runa, mas queria mais promocoes de cassino que hipnotizam. No fim das contas, SpellWin Casino e um cassino online que e um portal de diversao para os magos do cassino! Alem disso a interface do cassino e fluida e brilha como uma pocao reluzente, adiciona um toque de encantamento ao cassino.
spellwin online|
Estou completamente vidrado por BetorSpin Casino, e um cassino online que gira como um asteroide em chamas. Tem uma chuva de meteoros de jogos de cassino irados, com slots de cassino tematicos de espaco sideral. O servico do cassino e confiavel e brilha como uma galaxia, respondendo mais rapido que uma explosao de raios gama. Os pagamentos do cassino sao lisos e blindados, as vezes mais recompensas no cassino seriam um diferencial astronomico. No fim das contas, BetorSpin Casino e o point perfeito pros fas de cassino para os amantes de cassinos online! Alem disso a navegacao do cassino e facil como uma orbita lunar, torna a experiencia de cassino uma viagem espacial.
betorspin gГјncel giriЕџ|
sportwetten system strategie
My site … Online Wetten Verboten
doppelte chance kombiwette
Stop by my webpage – beste wett app österreich (Diana)
Estou completamente apaixonado por PlayPIX Casino, proporciona uma aventura pulsante. Ha uma explosao de jogos emocionantes, oferecendo jogos de mesa envolventes. 100% ate €500 + rodadas gratis. O servico esta disponivel 24/7, oferecendo respostas claras. O processo e simples e elegante, no entanto ofertas mais generosas seriam bem-vindas. Resumindo, PlayPIX Casino garante diversao constante para entusiastas de jogos modernos ! Alem disso o design e moderno e vibrante, adiciona um toque de conforto. Notavel tambem as opcoes variadas de apostas esportivas, fortalece o senso de comunidade.
Explorar o site|
https://bs2best.or.at
**breathe**
breathe is a plant-powered tincture crafted to promote lung performance and enhance your breathing quality.
I’d like to find out more? I’d care to find out some additional information.
Stop by my web-site … xem xxx miễn phí
tipp sportwetten
Also visit my homepage beste e wallet wettanbieter (Mable)
bs2web at Готов ли ты раскрыть тайны, окутывающие тёмную сеть? Blacksprut — это не просто бренд, это гарантия конфиденциальности, молниеносной скорости и абсолютной безопасности. Посети bs2best.at и узнай то, о чём остальные предпочитают не упоминать. Тебе откроются все тайны, тщательно скрываемые от посторонних глаз. Только для избранных, обладающих особым знанием. Не оставляя следов. Без каких-либо компромиссов. Только Blacksprut. Не упусти уникальный шанс оказаться в авангарде — bs2best.at уже ждёт тех, кто стремится к открытиям. Сможешь ли ты осмелиться познать истинное положение вещей?
beste wettbüro
Here is my web page; wetten bonus Code
Портал о строительстве домов https://doma-land.ru проекты и сметы, сравнение технологий (каркас, газобетон, кирпич, брус), фундамент и кровля, инженерия и утепление. Калькуляторы, чек-листы, тендер подрядчиков, рейтинги бригад, карта цен по регионам, готовые ведомости материалов и практика без ошибок.
halbzeit endstand wette doppelte chance wetten erklärung (Nicolas)
kombiwette mehrweg rechner
my webpage – sportwetten Vorhersagen app
Квартира с отделкой https://новостройкивспб.рф экономия времени и предсказуемый бюджет. Фильтруем по планировкам, материалам, классу дома и акустике. Проверяем стандарт отделки, толщину стяжки, ровность стен, работу дверей/окон, скрытые коммуникации. Приёмка по дефект-листу, штрафы за просрочку.
Tenho uma paixao ardente por PlayPIX Casino, transporta para um mundo de folia cativante. A variedade de titulos e deslumbrante, com sessoes ao vivo dinamicas. Eleva a diversao do jogo. O acompanhamento e impecavel, sempre pronto para ajudar. Os saques sao rapidos como um raio, as vezes bonus mais variados seriam bem-vindos. No geral, PlayPIX Casino e uma plataforma que reina suprema para jogadores em busca de adrenalina ! Alem disso a interface e fluida e elegante, adiciona um toque de conforto. Outro destaque o programa VIP com 5 niveis exclusivos, fortalece o senso de comunidade.
Descobrir|
**pinealxt**
pinealxt is a revolutionary supplement that promotes proper pineal gland function and energy levels to support healthy body function.
**energeia**
energeia is the first and only recipe that targets the root cause of stubborn belly fat and Deadly visceral fat.
продвижение сайтов топ 10 компаний [url=https://reiting-seo-agentstv.ru/]https://reiting-seo-agentstv.ru/[/url] .
**boostaro**
boostaro is a specially crafted dietary supplement for men who want to elevate their overall health and vitality.
**prostabliss**
prostabliss is a carefully developed dietary formula aimed at nurturing prostate vitality and improving urinary comfort.
Hello! I know this is kinda off topic however , I’d figured I’d
ask. Would you be interested in trading links or maybe guest authoring a blog article or vice-versa?
My website covers a lot of the same topics as yours
and I believe we could greatly benefit from each other.
If you’re interested feel free to shoot me an email. I look forward to hearing from you!
Fantastic blog by the way!
Rynek kasyn online w Polsce charakteryzuje się szybkim wzrostem Młodzi dorośli korzystający z urządzeń mobilnych napędzają wzrost popularności kasyn online oferujących bonus powitalny. Totalizator Sportowy posiada monopol, regulowany na mocy Ustawy o Grach Hazardowych z 2009 roku, a Total Casino jest wiodącą platformą. Konkurencyjny krajobraz obejmuje krajowych i zagranicznych operatorów posiadających licencje z UE. Zaawansowane technologie, takie jak integracja z krupierami na żywo i optymalizacja mobilna, poprawiają doświadczenia użytkowników, podczas gdy wybór kasyna online staje się coraz bardziej złożony inicjatywy odpowiedzialnego hazardu zyskują na znaczeniu. Dowiedz się więcej o dynamice rynku i przyszłych perspektywach.
wett tipp heute
Have a look at my web blog :: erfolgreiche wettstrategien
Поисковое продвижение помогает компаниям стать ближе к своим клиентам. Благодаря SEO пользователи находят нужные товары и услуги быстрее, а сайт получает больше трафика и заказов. Это инструмент, который работает на перспективу. Со временем видимость растёт, а вместе с ней и доход. Узнайте больше о пользе SEO – https://mediawiki.salesianos.es/index.php?title=Продвижение_Сайтов_С_Оплатой_За_Результат
seo services ranking [url=http://top-10-seo-prodvizhenie.ru]http://top-10-seo-prodvizhenie.ru[/url] .
раскрутка сайтов в москве в топ 10 [url=http://seo-prodvizhenie-reiting-kompanij.ru/]http://seo-prodvizhenie-reiting-kompanij.ru/[/url] .
die besten sportwetten tipps
Here is my website: Wettstrategien Livewetten
Estou completamente vidrado por SupremaBet Casino, da uma energia de cassino que e puro dominio. Tem uma tempestade de jogos de cassino irados, com jogos de cassino perfeitos pra criptomoedas. A equipe do cassino entrega um atendimento que e puro reinado, com uma ajuda que brilha como uma coroa. As transacoes do cassino sao simples como um mandato, de vez em quando mais giros gratis no cassino seria uma loucura. Em resumo, SupremaBet Casino garante uma diversao de cassino que e uma supremacia para os amantes de cassinos online! Alem disso o design do cassino e um espetaculo visual majestoso, o que torna cada sessao de cassino ainda mais majestosa.
supremabet – suprema bet ltda|
Ich bin suchtig nach Lapalingo Casino, es fuhlt sich an wie ein wilder Ritt durch die Spielwelt. Der Katalog des Casinos ist ein Kaleidoskop des Spa?es, inklusive stilvoller Casino-Tischspiele. Der Casino-Service ist zuverlassig und glanzend, ist per Chat oder E-Mail erreichbar. Casino-Zahlungen sind sicher und reibungslos, dennoch wurde ich mir mehr Casino-Promos wunschen, die explodieren. Kurz gesagt ist Lapalingo Casino ein Online-Casino, das wie ein Sturm begeistert fur Fans von Online-Casinos! Zusatzlich die Casino-Seite ist ein grafisches Meisterwerk, das Casino-Erlebnis total elektrisiert.
lapalingo no deposit bonus 2022|
Adoro o clima insano de DiceBet Casino, parece um tornado de diversao. Tem uma enxurrada de jogos de cassino variados, incluindo jogos de mesa de cassino cheios de classe. O atendimento ao cliente do cassino e uma maravilha, garantindo suporte de cassino direto e na lata. Os saques no cassino sao velozes como um cometa, mesmo assim mais giros gratis no cassino seria uma loucura. No fim das contas, DiceBet Casino garante uma diversao de cassino que e um estouro para os amantes de cassinos online! E mais a navegacao do cassino e facil como brincadeira, o que deixa cada sessao de cassino ainda mais animal.
dicebet casino|
**potentstream**
potentstream is engineered to promote prostate well-being by counteracting the residue that can build up from hard-water minerals within the urinary tract.
купить мухоморы Магазин muhomorus в интернете предлагает возможность купить мухоморы с доставкой в любую точку России. Предлагаем выгодные цены на экологически чистую продукцию, предназначенную для снятия тревожных состояний, стресса, депрессии, хронической усталости, а также для облегчения признаков различных заболеваний. Подчеркиваем, что сушеные мухоморы не являются лекарством, а относятся к парафармацевтике – альтернативному средству, применяемому каждым человеком по собственному желанию в качестве дополнительной терапии. Законность сбора, сушки, продажи и покупки гарантируется. У нас вы можете законно приобрести микродозы.
радіоактивні ізотопи це
SEO — это один из самых надёжных инструментов привлечения клиентов из интернета. Он помогает улучшить позиции сайта, увеличить посещаемость и повысить доверие аудитории. Такой подход особенно эффективен для компаний, которые хотят развиваться без больших рекламных затрат. Регулярная оптимизация даёт стабильный результат. Подробнее об этом читайте: заказать сео сайта
Выбор Идеального TikTok Mod: Последняя Версия, Русский Язык и Безопасность Чтобы получить максимальную отдачу от TikTok мода, важно выбирать последнюю версию, которая содержит все новейшие функции и исправления ошибок. Запросы “тикток мод последняя версия 2025” и “скачать тикток мод последняя версия” это подтверждают. Кроме того, для многих пользователей важна поддержка русского языка, поэтому “русский тикток мод” и “тикток мод русская версия” также пользуются популярностью. Но, прежде всего, необходимо помнить о безопасности. Перед установкой мода обязательно проверьте его на наличие вирусов и других вредоносных программ. Используйте надежные антивирусные сканеры и читайте отзывы других пользователей. Только так можно наслаждаться всеми преимуществами модифицированного TikTok, не подвергая риску свое устройство и личные данные. тикток мод тг
I’m truly enjoying the design and layout of your website.
It’s a very easy on the eyes which makes itt much more pleasant ffor me to come here andd visit more often. Did
yoou hire out a degeloper tto cteate your theme?
Great work!
SEO-продвижение помогает компаниям оставаться конкурентоспособными. Оптимизация улучшает позиции сайта, увеличивает конверсию и укрепляет доверие клиентов. Регулярная работа над контентом и аналитикой обеспечивает стабильный результат. Это основа успешного бизнеса в интернете. Узнайте больше о SEO – https://wikigranny.com/wiki/index.php/Продвижение_Сайтов_Для_Роста_Посещаемости
скачать самый мод тик ток Этапы Установки и Потенциальные Риски: Баланс Между Функциональностью и Безопасностью Процесс установки TikTok мода зависит от вашего устройства (Android или iPhone). Обычно требуется удаление оригинальной версии TikTok и установка APK-файла (для Android) или использование сторонних инструментов для iOS. Необходимо помнить, что использование модов может нарушать условия использования TikTok и привести к блокировке аккаунта. Запросы “как установить мод на тик ток” и “как сделать тик ток мод” отражают стремление пользователей к самостоятельной настройке и созданию кастомных версий.
Estou completamente apaixonado por BETesporte Casino, proporciona uma aventura eletrizante. Ha uma explosao de jogos emocionantes, com slots modernos e tematicos. O bonus de boas-vindas e empolgante. O suporte ao cliente e excepcional, garantindo atendimento de alto nivel. O processo e simples e direto, no entanto mais apostas gratis seriam incriveis. Em sintese, BETesporte Casino e uma plataforma que domina o campo para quem usa cripto para jogar ! Alem disso a interface e fluida e energetica, instiga a prolongar o jogo. Igualmente impressionante o programa VIP com niveis exclusivos, proporciona vantagens personalizadas.
Aprender como|
Excellent blog right here! Additionally your web site quite a bit up fast!
What web host are you using? Can I get your affiliate hyperlink to your host?
I wish my website loaded up as quickly as yours lol
SEO-продвижение помогает сайту привлекать новых клиентов и укреплять доверие аудитории. Работа с контентом, структурой и техническими настройками ресурса повышает позиции и улучшает опыт пользователей. Регулярная аналитика и корректировка стратегии обеспечивают стабильный рост и долгосрочный эффект. Такой подход делает сайт надёжным источником клиентов. Читайте подробнее – https://cbaaacademy.com/2025/10/создание-и-продвижение-сайтов-в-москв/
SEO-продвижение даёт возможность повысить видимость сайта в интернете и привлечь новых клиентов. Грамотная работа с контентом, структурой страниц и техническими настройками улучшает качество ресурса. Пользователи находят сайт быстрее, а поисковые системы оценивают его выше. Регулярное обновление информации и мониторинг конкурентов поддерживают стабильные результаты. Такой подход обеспечивает долгосрочный эффект и развитие компании без лишних расходов. Читайте подробнее по ссылке https://flynonrev.com/airlines/index.php/Доступное_Продвижение_Сайта_Для_Малого_Бизнеса
eurovision wetten deutschland
Also visit my site :: wettbüRo ludwigshafen
впн для соцсетей VPN: Ключ к YouTube без ограничений и беспрепятственному доступу к любимым развлечениям и информации. Забудьте о надоедливых сообщениях о недоступности контента в вашем регионе. VPN для YouTube (часто именуемый “ютуба”) откроет вам мир безграничных возможностей, позволяя наслаждаться любимыми видео и каналами без каких-либо ограничений. Для любителей онлайн-игр VPN для игр снизит пинг и защитит от DDoS-атак, обеспечивая комфортный игровой процесс. VPN для социальных сетей гарантирует приватность и безопасность вашей онлайн-активности, защищая от слежки и цензуры. Пользователям из России предлагается российский VPN, обеспечивающий высокую скорость и стабильность соединения.
https://baidak.ru/
установка кондиционера в москве недорого Установка сплит системы: Профессиональный Монтаж для Эффективной Работы Сплит-системы – популярный выбор для кондиционирования помещений благодаря своей эффективности и тихой работе. Наша команда опытных специалистов выполнит установку сплит системы в соответствии со всеми техническими требованиями, обеспечивая ее надежную и долговечную работу. Мы используем только качественные материалы и современное оборудование, чтобы гарантировать безупречный результат.
sportwetten bester anbieter
Take a look at my page: sportwette ohne oasis (Bradley)
Девушка-мастер просто красавица, с утончёнными чертами лица и обворожительной улыбкой. Массаж чувственный, мягкий, расслабляющий, каждое движение продуманное и деликатное. Атмосфера салона уютная и комфортная. Очень советую, проститутки недорого нск, https://sibirka.com/. Был в восторге, сервис просто супер.
Hi to all, how is all, I think every one is getting more from this website, and your views are fastidious in favor of new visitors.
**hepatoburn**
hepatoburn is a premium nutritional formula designed to enhance liver function, boost metabolism, and support natural fat breakdown.
Компания «СибЗТА» https://sibzta.su производит задвижки, клапаны и другую трубопроводную арматуру с 2014 года. Материалы: сталь, чугун, нержавейка. Прочные уплотнения, стандарты ГОСТ, индивидуальные решения под заказ, быстрая доставка и гарантия.
Ich liebe den Zauber von Trickz Casino, es bietet ein Casino-Abenteuer, das wie eine Illusion verblufft. Die Spielauswahl im Casino ist wie ein Zauberkoffer voller Wunder, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Die Casino-Mitarbeiter sind schnell wie ein Kaninchen aus dem Hut, sorgt fur sofortigen Casino-Support, der begeistert. Casino-Gewinne kommen wie ein Blitz aus dem Hut, aber die Casino-Angebote konnten gro?zugiger sein. Kurz gesagt ist Trickz Casino ein Muss fur Casino-Fans fur Fans moderner Casino-Slots! Nebenbei die Casino-Seite ist ein grafisches Meisterwerk, einen Hauch von Zauberei ins Casino bringt.
trickz casino recension|
Ich bin komplett hin und weg von SpinBetter Casino, es erzeugt eine Spielenergie, die fesselt. Es gibt eine unglaubliche Auswahl an Spielen, mit aufregenden Sportwetten. Der Kundenservice ist ausgezeichnet, mit praziser Unterstutzung. Die Zahlungen sind sicher und smooth, dennoch mehr Rewards waren ein Plus. In Kurze, SpinBetter Casino garantiert hochsten Spa? fur Adrenalin-Sucher ! Au?erdem das Design ist ansprechend und nutzerfreundlich, was jede Session noch besser macht. Ein Pluspunkt ist die Vielfalt an Zahlungsmethoden, die Flexibilitat bieten.
https://spinbettercasino.de/|
Ich bin total begeistert von Billy Billion Casino, es fuhlt sich an wie eine unwiderstehliche Energie fur Spieler. Die Auswahl ist reich und vielfaltig, mit immersiven Live-Casino-Sessions von Evolution Gaming. Der Kundenservice ist erstklassig, bietet klare und hilfreiche Antworten. Der Auszahlungsprozess ist einfach und zuverlassig, trotzdem die Boni wie der 100%-Willkommensbonus bis 7500 € konnten ofter kommen. Zusammengefasst ist Billy Billion Casino bietet ein sicheres und faires Spielerlebnis mit einem Sicherheitsindex von 8,5 fur die, die gerne wetten! Erganzend das Design ist visuell ansprechend und einzigartig, einen Hauch von Eleganz hinzufugt.
billy billion casino login|
kraken marketplace
кракен клиент
wetten vorhersagen app
Also visit my webpage … die besten wettstrategien (Wilbur)
мелбет промокод на фрибет при регистрации
beste buchmacher sportwetten
Feel free to visit my webpage … Die Besten Wett Apps,
https://Decanitourism.Org,
Thank you for the auspicious writeup. It in truth was a entertainment account it. Look complex to more added agreeable from you! By the way, how could we be in contact?
https://staffinggoals.com
**hepatoburn**
hepatoburn is a potent, plant-based formula created to promote optimal liver performance and naturally stimulate fat-burning mechanisms.
wette deutschland
My site: geld zurück online sportwetten, Rebbeca,
Hondrolife und die Unterstützung des Muskelaufbaus
Um die Kraft unseres Körpers gezielt zu steigern, ist es von großer Bedeutung, die richtigen Mittel und Methoden anzuwenden. Viele Menschen streben danach, ihre körperliche Fitness zu verbessern, um fit und gesund zu bleiben. Dabei spielt die Auswahl geeigneter Supplements eine wesentliche Rolle. Sie können den Körper darin fördern, effektiv Muskeln aufzubauen und sich schneller zu regenerieren. Eine ausgewogene Ernährung allein reicht oft nicht aus, weshalb gezielte Ergänzungen von Nutzen sein können.
Es gibt eine Vielzahl an Produkten auf dem Markt, die versprechen, den Aufbau von Muskelmasse zu unterstützen. Einige dieser Mittel belegen ihre Wirksamkeit durch wissenschaftliche Studien, während andere auf der Erfahrung von Sportlern basieren. Wichtig ist, dass die Inhaltsstoffe sorgfältig ausgewählt werden, um die gewünschten Ergebnisse zu erzielen. Außerdem darf man die Bedeutung von Training und Regeneration nicht unterschätzen, denn sie sind ebenfalls entscheidend für den Fortschritt.
Nachhaltigkeit und Gesundheit stehen im Vordergrund. Wer seine Leistung steigern möchte, sollte auch auf die Qualität der Produkte achten. Die richtige Kombination aus einer proteinschweren Ernährung, intensiven Trainingseinheiten sowie hochwertigen Ergänzungsmitteln kann die Leistung erheblich steigern. Ein bewusster Umgang mit diesen Faktoren führt nicht nur zu sichtbaren Ergebnissen, sondern stärkt auch das Wohlbefinden insgesamt.
https://hondrolife.biz/ für Muskelregenerierung
Nach intensiven Trainingseinheiten sind die Muskeln erschöpft. Die Regeneration spielt eine entscheidende Rolle für den anschließenden Fortschritt. Es geht darum, dem Körper die nötige Pflege zukommen zu lassen. Die richtige Nahrungsergänzung kann hier großen Unterschied machen. Sie unterstützt den natürlichen Heilungsprozess.
Ein Produkt, das diese Aspekte angeht, fördert die Erneuerung der Muskelzellen auf effektive Weise und trägt dazu bei, die Erholungszeit zu verkürzen. Wenn man bedenkt, dass die Regeneration nicht nur wichtig für das Wohlbefinden ist, sondern auch für die Leistungssteigerung, wird schnell klar, wie bedeutend diese Phase eines Trainingszyklus ist.
Energie und Nährstoffe, die nach dem Training benötigt werden, sichern die optimale Funktionsweise der Muskulatur. Dazu zählen Aminosäuren, die essentielle Bausteine für den Eiweißaufbau darstellen. Ein regenerationsförderndes Produkt kann die Muskelregeneration erheblich verbessern, indem es diese wertvollen Komponenten bereitstellt. Die Ergebnisse sind oft spürbar schneller sichtbar und motivieren für zukünftige Trainingseinheiten.
Zusätzlich verfügt ein solches Mittel über Eigenschaften, die Entzündungen reduzieren können. So wird die Erholung nicht nur gefördert, sondern auch Beschwerden nach dem Sport minimiert. Wer sich um die eigene Gesundheit kümmert, investiert in langfristige Fortschritte. Das richtige Produkt liefert alles, was der Körper nach körperlicher Anstrengung benötigt.
Das Ziel ist eine harmonische Balance zwischen Belastung und Erholung. Die Muskulatur kann sich regenerieren. Schließlich ist es das, was erst den Fortschritt ermöglicht und die Fitness langfristig steigert. Daher sollte man unbedingt auf eine qualitativ hochwertige Nahrungsergänzungsmittel setzen, die die Erholung unterstützt.
Die Rolle von Hondrolife im Fitnessprogramm
Ein spezialisierter Ansatz kann den Fortschritt im Sport erheblich beschleunigen. Dabei spielen verschiedene Produkte eine entscheidende Rolle. Eines davon wird häufig übersehen, ist aber unverzichtbar. Es trägt nicht nur zur Verbesserung der allgemeinen Fitness bei, sondern fördert auch spezifische Ergebnisse.
Die Integration in den Trainingsplan kann transformative Effekte haben. Ein effektives Supplement kann helfen, die Leistungsgrenzen zu überwinden. Zudem beeinflusst es das Wohlbefinden positiv, was die Motivation steigert. Ein angenehmes Gefühl im Körper ist der Schlüssel zur langfristigen Fitness. Das bringt mehr Freude an der Bewegung und sorgt für bessere Ergebnisse.
Ein gut durchdachter Zeitplan für die Einnahme ist essenziell. Wer gezielt auf seine Ziele hinarbeitet, wird schnell Verbesserungen feststellen. Die Synergie zwischen Training und Nahrungsergänzung maximiert den Nutzen. Auch der psychologische Effekt sollte nicht unterschätzt werden. Ein gewisses Maß an Sicherheit und Unterstützung kann Wunder wirken.
Insgesamt bietet dieses Produkt nicht nur eine Basis für Fortschritt, sondern weckt auch das Potenzial eines jeden Sportlers. Damit wird nicht nur der Körper gestärkt, sondern auch der Geist. Die sportliche Reise wird somit zu einem erfüllenden Erlebnis, das über körperliche Veränderungen hinausgeht.
купить красный мухомор На сайте muhomorus вы можете оформить заказ на мухоморы с доставкой по всей территории РФ. У нас выгодные предложения на экологически чистые продукты, которые помогут вам справиться с тревожностью, стрессом, депрессией, хронической усталостью и облегчат симптомы различных заболеваний. Необходимо отметить, что сушеные мухоморы не относятся к лекарственным средствам, их классифицируют как парафармацевтику, которая является альтернативным средством, используемым по личному усмотрению в качестве вспомогательной терапии. Все этапы – сбор, сушка, продажа и покупка – осуществляются абсолютно законно. Мы предлагаем вам приобрести микродозинг на законных основаниях.
Great post. Just a heads up – I am running Ubuntu with the beta of Firefox and the navigation of your blog is kind of broken for me.
**flow force max**
flow force max delivers a forward-thinking, plant-focused way to support prostate health—while also helping maintain everyday energy, libido, and overall vitality.
https://socialmeet.app/read-blog/27303
The post is absolutely great! Lots of great info and inspiration, both of which we all need! Also like to admire the time and effort you put into your blog and detailed information you offer! I will bookmark your website!
**prodentim**
prodentim is a forward-thinking oral wellness blend crafted to nurture and maintain a balanced mouth microbiome.
список seo агентств [url=https://reiting-seo-kompaniy.ru/]reiting-seo-kompaniy.ru[/url] .
An outstanding share! I’ve just forwarded this onto a coworker who has been doing a little homework on this. And he in fact ordered me dinner due to the fact that I found it for him… lol. So allow me to reword this…. Thanks for the meal!! But yeah, thanx for spending the time to discuss this matter here on your web page.
easystaffingmd.com
**cellufend**
cellufend is a natural supplement developed to support balanced blood sugar levels through a blend of botanical extracts and essential nutrients.
**revitag**
revitag is a daily skin-support formula created to promote a healthy complexion and visibly diminish the appearance of skin tags.
**neuro genica**
neuro genica is a dietary supplement formulated to support nerve health and ease discomfort associated with neuropathy.
https://www.calgarynews.net/newsr/15798
Je suis totalement enflamme par VBet Casino, c’est un casino en ligne qui jaillit comme un volcan en furie. Il y a une deferlante de jeux de casino captivants, proposant des slots de casino a theme volcanique. Le personnel du casino offre un accompagnement digne d’un volcan, repondant en un eclair brulant. Le processus du casino est transparent et sans cendres, par moments les offres du casino pourraient etre plus genereuses. Pour resumer, VBet Casino offre une experience de casino ardente pour les joueurs qui aiment parier avec panache au casino ! Bonus le site du casino est une merveille graphique ardente, ajoute une touche de feu au casino.
диво колесо vbet|
Ich bin total begeistert von Lowen Play Casino, es verstromt eine Spielstimmung, die wie eine Savanne tobt. Der Katalog des Casinos ist ein Dschungel voller Nervenkitzel, inklusive stilvoller Casino-Tischspiele. Die Casino-Mitarbeiter sind schnell wie ein Gepard, mit Hilfe, die wie ein Brullen wirkt. Casino-Transaktionen sind simpel wie ein Pfad im Busch, ab und zu mehr Casino-Belohnungen waren ein koniglicher Gewinn. Zusammengefasst ist Lowen Play Casino eine Casino-Erfahrung, die wie ein Lowe glanzt fur Fans von Online-Casinos! Zusatzlich die Casino-Plattform hat einen Look, der wie eine Savanne funkelt, den Spielspa? im Casino in die Hohe treibt.
gehalt bezirksleiter löwen play|
Ich bin abhangig von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Es wartet eine Fulle spannender Optionen, mit Spielen, die fur Kryptos optimiert sind. Die Hilfe ist effizient und pro, mit praziser Unterstutzung. Die Zahlungen sind sicher und smooth, trotzdem mehr abwechslungsreiche Boni waren super. Global gesehen, SpinBetter Casino ist eine Plattform, die uberzeugt fur Casino-Liebhaber ! Zusatzlich die Navigation ist kinderleicht, verstarkt die Immersion. Hervorzuheben ist die Sicherheit der Daten, die Vertrauen schaffen.
https://spinbettercasino.de/|
Galera, preciso contar o que achei sobre o Bingoemcasa porque achei muito alem do que esperava. O site tem um clima acolhedor que lembra um salao cheio de energia. As salas de bingo sao cheias de energia, e ainda testei uns joguinhos extras, todos vieram com graficos bonitos. O atendimento no chat foi respondeu em segundos, o que ja me deixou confiante. As retiradas foram eficientes de verdade, inclusive testei cartao e funcionou perfeito. Se pudesse apontar algo, diria que senti falta de ofertas extras, mas nada que estrague a experiencia. Resumindo, o Bingoemcasa foi uma otima descoberta. Com certeza vou continuar jogando
bingoemcasa|
kraken ссылка Остерегайтесь Подделок: Идентификация Истинного Ключа С ростом популярности “Кракена” множатся и подделки, зеркала-призраки, созданные для обмана и вымогательства. “Kraken,” – это имя стало брендом, который пытаются присвоить мошенники, жаждущие наживы. Важно помнить: истинная “кракен ссылка” всегда ускользает, ее приходится искать в проверенных источниках, в надежных кругах, где репутация ценится превыше всего. Не доверяйте первому встречному, перепроверяйте информацию, используйте многофакторную аутентификацию. Безопасность – ваш главный союзник в этом опасном путешествии.
Pretty! This has been an extremely wonderful article.
Many thanks for supplying these details.
Hi there, You have done an incredible job. I will certainly digg it and personally recommend to my friends.
I am sure they’ll be benefited from this web site.
Unlock your images with WebPtoJPGHero.com, the simple, free solution for solving WebP compatibility issues. While the WebP format helps websites load faster, it can leave you with files you can’t easily edit or share. Our online tool provides an instant fix, transforming any WebP image into a high-quality JPG that works seamlessly across all your devices and applications. You can fine-tune the output quality to balance clarity and file size, and even save time by converting an entire batch of photos at once. It’s all done quickly and privately right in your browser — no software required.
I am curious to find out what blog platform you’re utilizing?
I’m having some small security issues with my latest
site and I would like to find something more safe.
Do you have any solutions?
Every weekend i used to pay a quick visit this site, as i wish
for enjoyment, as this this web site conations actually nice funny material too.
трансы Екатеринбург Этика Онлайн-Присутствия: Уважение, Конфиденциальность и Ответственность Эффективность и безопасность этих каналов напрямую зависят от ответственного поведения каждого участника. Уважение к приватности, недопустимость дискриминации и поддержание атмосферы взаимопонимания – ключевые принципы здорового онлайн-сообщества. Telegram-каналы о трансгендерности – это не просто место для обмена информацией, это пространство, где формируется идентичность, находится поддержка и прокладываются мосты между людьми.
beste wett tipps kostenlos
Feel free to visit my web site :: Schweiz wetten
Greetings from Ohio! I’m bored to tears at work so I decided to check out your blog on my
iphone during lunch break. I really like the information you provide here
and can’t wait to take a look when I get home. I’m amazed at how fast your blog loaded on my cell phone
.. I’m not even using WIFI, just 3G .. Anyhow, excellent site!
DRINKIO — лучший вариант для тех, кто ценит комфорт и оперативность. Доставка всегда вовремя, даже в позднее время. Операторы доброжелательные, всё объясняют и уточняют детали. Курьер приехал быстро, всё аккуратно упаковано. Очень доволен обслуживанием https://drinkio105.ru/
Срочно нужно заменить лобовое стекло? Наша услуга по замене – это быстрое и качественное решение. Мы используем только оригинальные стекла и современные материалы, чтобы обеспечить идеальную геометрию, герметичность и сохранение всех функций: заменить лобовое стекло
Terrific work! That is the kind of information that should be shared around the web.
Shame on Google for now not positioning this publish upper!
Come on over and talk over with my website .
Thank you =)
An outstanding share! I have just forwarded
this onto a colleague who has been doing a little homework on this.
And he actually ordered me dinner due to the fact that I found it for him…
lol. So allow me to reword this…. Thanks for the meal!!
But yeah, thanx for spending time to discuss this issue here on your site.
Yeni başlayanlar üçün kupon düzəltmə bələdçisi aydın yazılıb, hər addımda yardım pəncərələri çıxır.
Əlavə təkliflərdə futbol, tennis, basketbol və e-idman üçün gündəlik artırılmış əmsallar var. Android istifadəçiləri [url=https://pinco-casino-azerbaijan.biz.ua/]pinco yüklə[/url] bələdçisini izləyərək apk-ni bir dəqiqəyə quraşdıra bilər. AZN balansı aydın görünür, tranzaksiya tarixi filtrə ilə rahat axtarılır. Minimum və maksimum mərc diapazonları həm yeni başlayanlar, həm də high-rollerlər üçün uyğundur.
Futbol üçün xG, şans yaradıcılığı və pressinq göstəricilərinə baxmaq faydalıdır. iOS istifadəçiləri üçün PWA veb-tətbiqi ikonaya əlavə etməklə bir toxunuşda açılır.
Təhlükəsizlik bildirişləri qeyri-adi giriş zamanı dərhal göndərilir. Uşaqların müdafiəsi ilə bağlı xəbərdarlıq bannerləri görünən yerdədir. Kampaniya təqvimi bonusları planlaşdırmağa və maksimum fayda götürməyə imkan verir.
Hmm is anyone else encountering problems with the images on this blog loading? I’m trying to figure out if its a problem on my end or if it’s the blog. Any feed-back would be greatly appreciated.
https://keslaser.com.ua/chym-vidrizniaietsia-pnevmoinstrument-vid-elektrychnoho-pry-remonti-far.html
Популярные https://mozillabd.science/wiki/Ruseriya_58a всегда в тренде, что сейчас смотрите?
1xbet g?ncel giri? [url=http://1xbet-giris-1.com]http://1xbet-giris-1.com[/url] .
Популярные https://wiki.anythingcanbehacked.com/index.php?title=Ruseriya_43I всегда держат в напряжении, рекомендую всем!
bonus wetten vergleich
my website; sportwetten paysafecard
wettbüro konstanz
My web-site – handicap live wette, https://www.relampagocacambas.Com.Br/,
I’d like to find out more? I’d love to find out more details.
Matç öncəsi statistik blokda komanda forması, H2H və zədəlilər siyahısı toplanıb.
Qeydiyyat e-mail və ya telefonla 1 dəqiqədən az çəkir, təsdiqdən sonra başlanğıc bonusu aktivləşir. Mobil quraşdırma üçün ən son paket [url=https://pinco-casino-azerbaijan.biz.ua/]pinco casino apk[/url] bölməsindədir; yenilənmə xəbərdarlıqları tətbiqdaxili gəlir. Çıxarışlar e-cüzdana adətən bir neçə dəqiqə, kartlara isə bankdan asılı olaraq daha uzun çəkir. Axtarış paneli ad, provayder və mexanika üzrə işləyir — nəticələr dərhal çıxır.
Slotlarda volatilite seçimi qısa və ya uzun sessiyalara görə dəyişdirilə bilər. Mobil interfeys jestlərlə işləyir, kupon sürüşdürmə ilə redaktə olunur.
Hesab bərpası SMS və ya e-poçt vasitəsilə aparılır. Bonus şərtlərində çevirmə tələbləri və maksimal uduş hədləri aydın göstərilir. Hərtərəfli filtr və axtarış sayəsində istədiyiniz məzmunu saniyələr içində tapırsınız.
https://ritual-uslugi-spb-reg.ru/
Bank kartı və e-cüzdanla depozitlər anında düşür, ödəniş limiti AZN üçün əlverişlidir.
Promokod bölməsinə daxil olmaq asandır, aktiv kodlar avtomatik tanınır. Daim eyni ünvanı yoxlayın: [url=https://pinco-casino-azerbaijan.biz.ua/]www.pinco-casino-azerbaijan.biz.ua/[/url]; pinco azerbaycan yukle və kampaniyalar yalnız burada etibarlıdır. Hesab təhlükəsizliyi üçün iki faktorlu giriş və cihazların siyahısı var. Top oyunlar və yeni relizlər ayrı vitrinlərdə təqdim edilir.
Oyun qaydaları hər masada ayrıca açılır və suallara cavab verir. Android apk yükləməsi rəsmi səhifədən olur və quraşdırma bələdçisi addım-addım göstərilir.
Azərbaycan dilində konsultasiya mövcuddur və texniki suallar tez həll olunur. Qaydalar və şərtlər sadə dildə tərtib edilib, əsas başlıqlar rahat tapılır. Yüksək əmsal axtaranlar üçün gündəlik yüksəldilmiş xətt ayrıca işarələnib.
צובט! כן, הוא צובט! “נטש! תביא את המגבת! ג ‘ ל רחצה נכנס התנהגתי מבולבלת, לא הרגשתי בנוח. חצי וגיששה את הזין והתחילה ללטף דרך מוסתר מעיניים סקרניות על ידי “לחיים”אלסטיות. היה צריך להפיץ את” important source
Kuponda multi-bet, sistem və tez düzəliş funksiyaları var, səhv əmsal da avtomatik düzəlir.
Çoxlu lokal ödəniş üsulu dəstəklənir, komissiyalar real vaxt göstərilir. Rəsmi domeni yadda saxlayın: [url=https://pinco-casino-azerbaijan.biz.ua/]pinco-casino-azerbaijan biz ua[/url]; pinco azerbaycan yükle təlimatı bu ünvandadır. Sürətli ödəniş üçün sevimli metodları saxlayıb bir kliklə istifadə etmək olur. Minimum və maksimum mərc diapazonları həm yeni başlayanlar, həm də high-rollerlər üçün uyğundur.
Yeni oyunçular üçün təlimat məqalələri sadə dildə yazılıb, ekran görüntüləri kömək edir. Biometrik giriş barmaq izi və ya Face ID ilə tez və təhlükəsizdir.
Təhlükəsizlik bildirişləri qeyri-adi giriş zamanı dərhal göndərilir. Məsuliyyətli oyun üçün yaş təsdiqi və limitlər məcburidir. Favoritlər siyahısı ilə sevdiyiniz liqalar və slotlar bir yerdə saxlanır.
sportwetten tipps für heute
Feel free to visit my page Wetten Spiel
hardman4schools – I’m impressed by the message clarity and strong sense of community\
wie funktionieren handicap online Wetten österreich
1xbet g?ncel giri? [url=https://1xbet-giris-7.com/]1xbet g?ncel giri?[/url] .
Canlı izləmə və canlı mərc ekranları yan-yana açılır, gecikmə minimumdur.
Promokod bölməsinə daxil olmaq asandır, aktiv kodlar avtomatik tanınır. Lokal ödənişlər və AZN balansı üçün [url=https://pinco-casino-azerbaijan.biz.ua/]pinco az[/url] bölməsinə keçin, limitsiz canlı mərc rahatdır. Depozit bonusunun qaydaları dildə qısa və misallarla izah edilib. Turnirlər slotlarda aktivliyi artırır və real hədiyyələr verir.
Canlı mərclərdə gecikməni nəzərə alıb əmsal kilidlərinə diqqət etmək lazımdır. Android apk yükləməsi rəsmi səhifədən olur və quraşdırma bələdçisi addım-addım göstərilir.
Messencer kanallarına keçidlər rəsmi səhifədə yerləşir. Bonus şərtlərində çevirmə tələbləri və maksimal uduş hədləri aydın göstərilir. Seçim etməkdə çətinlik varsa, tematik toplular — futbol mərcləri, slot hitləri və canlı oyunlar — kömək edir.
що означає ім’я мирослав
https://atelierdevosidees.loiret.fr/profiles/promo_code_9/activity
Discover the best PS2 games in Canada! A curated list of timeless classics, including action, RPGs, and sports titles. Relive the nostalgia of top PlayStation 2 hits loved by gamers: complete list of PS2 games
beste wett seite
Also visit my blog post: was bedeutet quote bei wetten
Новые https://cameradb.review/wiki/Ruseriya_57v онлайн — всегда жду чего-то крутого!
https://www.israelnews.net/newsr/15860
whoah this blog is excellent i love studying your articles.
Keep up the good work! You recognize, many individuals are hunting around for this info, you could help them greatly.
Heya i am for the primary time here. I came across this board
and I to find It really helpful & it helped me out much.
I hope to present something back and aid others like you helped
me.
J’aime l’ambiance numerique de Monte Cryptos Casino, ca transporte dans un monde virtuel. Le catalogue est opulent et divers, proposant des tables sophistiquees. Elevant l’experience de jeu. Les agents repondent avec une vitesse fulgurante, toujours pret a decoder. Les retraits sont rapides comme une transaction, de temps a autre des recompenses supplementaires seraient ideales. Pour conclure, Monte Cryptos Casino est une plateforme qui domine l’univers crypto pour les passionnes de sensations numeriques ! En bonus la plateforme est visuellement eblouissante, donne envie de prolonger l’aventure. Egalement cool les paiements securises en BTC/ETH, propose des avantages uniques.
Regarder de plus prГЁs|
Je suis seduit par Impressario Casino, ca offre un plaisir sophistique. La variete des titres est raffinee, offrant des sessions live sophistiquees. Amplifiant le plaisir de jeu. Le support client est impeccable, garantissant un support de qualite. Les gains arrivent sans delai, parfois plus de promos regulieres ajouteraient du piquant. En resume, Impressario Casino est un incontournable pour les amateurs pour les fans de casino en ligne ! Ajoutons que la plateforme est visuellement somptueuse, amplifie le plaisir de jouer. Particulierement interessant les evenements communautaires engageants, propose des avantages personnalises.
Poursuivre la lecture|
kraken ссылка Этика Безопасчности и Мораль Анонимности “Кракен” предоставляет возможность действовать вне рамок общепринятых норм, но вместе с такой свободой приходит и ответственность. Анонимность не должна быть оправданием для злоупотреблений, для причинения вреда другим. Помните о моральных принципах, даже когда никто не видит. Используйте “Kraken” с умом, с пониманием последствий, и тогда он станет не источником проблем, а инструментом для достижения собственных целей.
J’aime l’aura futuriste de Monte Cryptos Casino, on ressent une energie decentralisee. Il y a une profusion de jeux captivants, offrant des sessions en direct immersives. Avec des depots crypto instantanes. Le suivi est irreprochable, avec une aide precise et fiable. Le processus est lisse comme un wallet, cependant des bonus plus varies seraient un tresor. Pour conclure, Monte Cryptos Casino est une plateforme qui domine l’univers crypto pour les passionnes de sensations virtuelles ! Par ailleurs la plateforme est visuellement eblouissante, facilite une immersion totale. A souligner les options de paris variees, propose des avantages uniques.
Voir les nouveautГ©s|
J’ai une passion ardente pour Monte Cryptos Casino, ca procure un frisson virtuel unique. Le repertoire est riche et varie, proposant des jeux de table sophistiques. Boostant votre portefeuille initial. L’assistance est rapide et professionnelle, avec une aide precise et fiable. Les paiements sont fluides et fiables, de temps en temps des offres plus genereuses ajouteraient du prestige. En bref, Monte Cryptos Casino est une plateforme qui domine le jeu virtuel pour ceux qui parient avec des cryptos ! Ajoutons que le site est rapide et captivant, ce qui rend chaque session plus immersive. Particulierement captivant les options de paris variees, offre des recompenses continues.
Explorer les dГ©tails|
J’ai une passion fluviale pour BassBet Casino, ca offre un plaisir fluvial. La variete des titres est rapide, offrant des sessions live immersives. Le bonus de bienvenue est rapide. Le support est irreprochable, offrant des reponses claires. Le processus est simple et elegant, cependant des bonus plus varies seraient une vague. En bref, BassBet Casino vaut une peche excitante pour les fans de casino en ligne ! A noter le site est rapide et attractif, ajoute une touche de vitesse. Particulierement interessant le programme VIP avec des niveaux exclusifs, assure des transactions fiables.
https://bassbetcasinobonus777fr.com/|
J’adore l’atmosphere mystique de Spinit Casino, c’est une plateforme qui tourbillonne d’elegance. Le catalogue est riche et varie, comprenant des jeux compatibles avec les cryptos. Renforcant votre capital initial. Les agents repondent comme par magie, toujours pret a transformer. Les retraits sont fluides comme un tour de passe-passe, cependant des bonus plus varies seraient un tour. Au final, Spinit Casino garantit un plaisir constant pour les joueurs en quete d’excitation ! Par ailleurs l’interface est fluide comme un sort, donne envie de prolonger l’aventure. Un plus les options de paris sportifs variees, assure des transactions fiables.
https://casinospinitfr.com/|
Je suis accro a Spinit Casino, c’est une plateforme qui tourne comme un conte. Le catalogue est riche et varie, incluant des paris sportifs dynamiques. Amplifiant le plaisir de jeu. Le support client est enchante, joignable a toute heure. Les paiements sont securises et rapides, cependant quelques tours gratuits supplementaires seraient bienvenus. Dans l’ensemble, Spinit Casino vaut une lecture magique pour ceux qui aiment parier en crypto ! En bonus la navigation est simple et magique, ce qui rend chaque session plus enchantee. A souligner les paiements securises en crypto, renforce le sentiment de communaute.
spinitcasinologinfr.com|
Je suis emerveille par Olympe Casino, c’est une plateforme qui evoque l’Olympe. La variete des titres est divine, offrant des sessions live immortelles. Renforcant votre tresor initial. Le support client est olympien, avec une aide precise. Les paiements sont securises et fluides, parfois quelques tours gratuits en plus seraient divins. En bref, Olympe Casino garantit un plaisir divin pour les passionnes de jeux antiques ! Ajoutons que la plateforme est visuellement olympienne, facilite une immersion totale. Egalement remarquable les paiements securises en crypto, qui booste l’engagement.
olympefr.com|
**sleep lean**
sleeplean is a US-trusted, naturally focused nighttime support formula that helps your body burn fat while you rest.
hardman4schools – I’m impressed by the message clarity and strong sense of community.
Pinco casino-da futbol və e-idman mərcləri üçün koeffisentlər tez-tez yenilənir, ona görə canlı rejimdə qərar vermək rahatdır.
Promokod bölməsinə daxil olmaq asandır, aktiv kodlar avtomatik tanınır. Yeganə düzgün keçid budur — [url=https://pinco-casino-azerbaijan.biz.ua/]PINCO-CASINO-AZERBAIJAN.BIZ.UA[/url]; pinco casino azerbaycan qaydaları burada açıqlanır. Məlumatlar TLS şifrələnməsi ilə qorunur, sessiya avtomatik vaxtı bitdikdə bağlanır. Demo rejimi ilə oyun mexanikasını risksiz yoxlamaq rahatdır.
Futbol üçün xG, şans yaradıcılığı və pressinq göstəricilərinə baxmaq faydalıdır. Bildirişlərdə favorit komandalara xüsusi əmsal artımları gəlir.
Təhlükəsizlik bildirişləri qeyri-adi giriş zamanı dərhal göndərilir. Region qaydalarına uyğun lokal variantlar və hüquqi məlumat verilir. Reytinqlər və istifadəçi rəyləri ən faydalı bölmələri tapmağa imkan verir.
займ деньги мфо микрозайм с моментальным одобрением
займ на карту без отказа https://zaimy-57.ru
срочно деньги займ https://zaimy-59.ru
кредитный займ срочно https://zaimy-61.ru
займ мгновенно https://zaimy-63.ru
получить займ деньги на карту без визита в офис
Cactus Casino — современная площадка для азартных игр
Любители азартных развлечений могут играть в казино Кактус на удобной и надежной платформе, которая сочетает стильный интерфейс, широкий ассортимент игр и быстрые выплаты. Сайт предлагает сотни популярных слотов, карточные и настольные игры, а также live-раздел с реальными дилерами, что делает игровой процесс живым и увлекательным.
Пользователи отмечают простоту регистрации и мгновенный доступ к играм, что особенно удобно для новичков, а опытные игроки ценят стабильность работы и разнообразие развлечений.
Бонусы казино и акции
Одним из ключевых преимуществ платформы являются бонусы казино, которые стимулируют активность игроков и увеличивают возможности для выигрыша.
• Приветственный пакет — до 80 000 рублей и 125 фриспинов в слоте Fortune of Giza;
• Промокоды для дополнительных бесплатных вращений;
• Система лояльности и регулярные турниры для постоянных участников.
Бонусная программа позволяет дольше наслаждаться слотами и другими играми, а также экономнее расходовать средства на игровой баланс.
Разнообразие игр и провайдеры
Cactus Casino https://kaktus-kazino.com сотрудничает с ведущими разработчиками: Pragmatic Play, BGaming, Wazdan, Spinomenal и Evoplay. Среди предложений:
• Классические и современные видеослоты;
• Автоматы с функцией Megaways;
• Карточные и настольные игры (блэкджек, баккара, рулетка);
• Live-шоу с настоящими ведущими;
• Прогрессивные джекпоты и эксклюзивные слоты.
Такой выбор позволяет каждому найти развлечение по вкусу и играть в слоты онлайн в безопасной и комфортной среде.
Пополнение счета и вывод средств
Платформа поддерживает различные способы оплаты:
1. СБП и Piastrix;
2. Банковские карты и Apple Pay/Google Pay;
3. Криптовалюты и FK Wallet;
4. Binance и другие электронные кошельки.
Минимальный депозит — от 350 рублей, минимальная сумма вывода — 1000 рублей. Большинство платежей обрабатывается в течение часа, что обеспечивает высокий уровень доверия со стороны пользователей.
Безопасность и лицензия
Cactus Casino работает по международной лицензии, что гарантирует честность игр и защиту личных данных. Все финансовые операции проходят шифрование, а результаты слотов и настольных игр проверяются сертифицированными провайдерами.
Итог: почему стоит выбрать Cactus Casino
Платформа сочетает надежность, комфорт и разнообразие развлечений. Простая регистрация, прозрачные условия бонусов, быстрые выплаты и широкий ассортимент слотов делают Cactus Casino привлекательным вариантом как для новичков, так и для опытных игроков.
Если вы ищете площадку для безопасной и интересной игры, официальный сайт Cactus Casino позволит насладиться азартом и шансом на выигрыши без лишних сложностей.
عزیز، در صورتی که به پلتفرمهای قمار تصور میکنید، توقف کنید.
اینجانب اتفاق خودم کردهام که نشان میگردد چنین سایتها ابزار برای گول زدن
و تخریب آینده هستند. سرمایه راحت از دست میگردد
و اعتیاد دائمی میشود. بهتر است اجتناب بمانید و از مشورت کارشناسان توجه شوید!
https://hd2k.ru/
betibet beste bonus sportwetten
Also visit my blog post: kombiwetten Vorhersagen (helpdesk.Templatemonsterdev.Com)
Смотреть https://heartkrafted.com/ruseriya-97d/ онлайн — мой способ отключиться от рутины!
createimpactonline – The platform’s analytics have provided insights to improve our strategies.
https://www.rusforum.com/group.php?do=discuss&group=&discussionid=785
partnershippowerhouse – A go-to resource for anyone looking to enhance their business alliances.
трансы Новосибириск Примеры и Особенности Каналов Каналы, подобные @ts_novosibirsk (трансы Новосибирск, трансы новосибирска), @ts_ekaterinburgh (трансы Екатеринбург, трансы Екатеринбурга), @ts_voronezh (трансы Воронеж, трансы Воронежа), @transy_volgograd (трансы Волгоград, трансы волгограда) и @ts_chelyabinska (трансы Челябинск, трансы челябинска), отражают специфику каждого города, собирая локальную информацию. Важно отметить, что контент этих каналов может быть разнообразным: от новостей и анонсов мероприятий до личных историй и дискуссий.
1xbet g?ncel [url=1xbet-4.com]1xbet-4.com[/url] .
1xbet giris [url=www.1xbet-giris-10.com/]1xbet giris[/url] .
https://kraken-shop24.com darknet
кракен вход
Also visit my web site: https://xn--krken-ucc.com
кредитный займ займы все
займ деньги сразу https://zaimy-69.ru
срочно нужен займ https://zaimy-71.ru
A thoughtful insight and ideas I will use on my blog. You’ve obviously spent some time on this. Congratulations
wetten online anbieter (Demetrius) deutschland schottland
united statesn roulette wheel play, free spins no deposit no wager uk 2021 and
best australia online casinos, or new no deposit free spins uk 2021
My homepage cool gambler names (Maxine)
Howdy, i read your blog from time to time and i own a similar one and i was just curious if you get a
lot of spam feedback? If so how do you stop it, any plugin or
anything you can recommend? I get so much lately it’s driving me insane
so any assistance is very much appreciated.
my web page :: are casino winnings considered
earned income – Gus,
займ мгновенно https://zaimy-76.ru
займы онлайн срочно https://zaimy-73.ru
займ без переплат https://zaimy-78.ru
кредитный займ срочно https://zaimy-80.ru
получить займ оформить займ без трудоустройства
займ без истории получить деньги без залога и справок
monumentremoval – The purpose is bold and the site feels deeply meaningful and timely.
Thank you for another fantastic post. Where else may just anyone get that type of info in such an ideal way of writing? I’ve a presentation next week, and I am at the search for such information.
официальный сайт драгон мани казино
I blog quite often and I genuinely appreciate your information. This great article has really peaked my interest.
I’m going to book mark your site and keep checking for new details about once
per week. I subscribed to your Feed too.
Look at my web blog: Web Page
https://xn--kr41-onb.com marketplace
https://www.shippingexplorer.net/en/user/robertreid/163553
денежный займ оформить микрозайм без отказа
What’s up, I desire to subscribe for this webpage to take hottest updates, therefore where can i do it please help.
казино зума
взять займ без отказа все займы онлайн на карту
займ без процентов https://zaimy-89.ru
kraken ios
Feel free to visit my webpage – https://xn--kr43-onb.com
https://gab.com/OtelloTravel/posts/114302667757671609
mystylecorner – Fast delivery, everything arrived intact, really appreciate the service today.
I am not positive where you are getting your information, but great topic.
I must spend some time studying more or figuring out more.
Thank you for great info I used to be searching for this info
for my mission.
Оформление декларации прошло без единой ошибки, сотрудники очень внимательные https://svobroker.ru/
Je suis seduit par Impressario Casino, c’est une plateforme qui evoque le raffinement. Il y a une abondance de jeux captivants, incluant des paris sportifs distingues. Le bonus de bienvenue est delicieux. Le support client est impeccable, joignable a toute heure. Les transactions sont fiables, cependant quelques tours gratuits supplementaires seraient bien venus. Pour conclure, Impressario Casino garantit un plaisir constant pour les fans de casino en ligne ! De plus la navigation est simple et gracieuse, donne envie de prolonger l’experience. Un plus les tournois reguliers pour la competition, renforce le sentiment de communaute.
Trouver la vГ©ritГ©|
J’ai une passion ardente pour Monte Cryptos Casino, c’est une plateforme qui pulse comme un reseau blockchain. Le catalogue est opulent et divers, comprenant des jeux optimises pour les cryptos. Elevant l’experience de jeu. L’assistance est precise et professionnelle, toujours pret a decoder. Les retraits sont rapides comme une transaction, neanmoins quelques tours gratuits en plus seraient bienvenus. Dans l’ensemble, Monte Cryptos Casino est un must pour les fans de blockchain pour les amateurs de casino en ligne ! Ajoutons que l’interface est fluide comme un flux de donnees, ajoute une touche de sophistication. Particulierement captivant les options de paris variees, propose des avantages uniques.
Voir tout|
Je suis charme par Impressario Casino, il procure une experience exquise. Les options sont vastes comme un menu etoile, incluant des paris sportifs distingues. 100% jusqu’a 500 € + tours gratuits. Les agents repondent avec courtoisie, joignable a toute heure. Les gains arrivent sans delai, cependant quelques tours gratuits supplementaires seraient bienvenus. Dans l’ensemble, Impressario Casino garantit un plaisir constant pour les amateurs de sensations elegantes ! En bonus le design est moderne et chic, ce qui rend chaque session plus raffinee. Un autre atout les tournois reguliers pour la competition, offre des recompenses continues.
AccГ©der au contenu|
J’aime l’aura futuriste de Monte Cryptos Casino, il offre une epopee chiffree. Les options sont vastes comme un reseau, comprenant des jeux tailles pour les cryptos. Le bonus d’entree est scintillant. Disponible 24/7 via chat ou email, toujours pret a decoder. Les paiements sont securises et fluides, neanmoins des bonus plus varies seraient un plus. En resume, Monte Cryptos Casino est une plateforme qui brille dans l’univers crypto pour les passionnes de sensations virtuelles ! Ajoutons que l’interface est fluide comme un flux de donnees, facilite une immersion totale. A souligner les evenements communautaires decentralises, propose des avantages uniques.
Visiter la plateforme|
Je suis completement electrise par BassBet Casino, ca transporte dans un univers de beats. Il y a un flot de jeux captivants, incluant des paris sportifs pleins de punch. 100% jusqu’a 500 € + tours gratuits. Le support client est au top, joignable a tout moment. Les transactions sont fiables, cependant quelques tours gratuits en plus seraient top. En resume, BassBet Casino est une plateforme qui fait vibrer pour les fans de casino en ligne ! Par ailleurs le design est moderne et lumineux, ajoute une touche de neon. Un point fort les evenements communautaires dynamiques, propose des avantages sur mesure.
bassbetcasinobonus777fr.com|
Je suis emerveille par Spinit Casino, il procure une experience rapide. Il y a une profusion de jeux excitants, proposant des jeux de table rapides. Le bonus de bienvenue est rapide. L’assistance est efficace et professionnelle, offrant des reponses claires. Les paiements sont securises et rapides, bien que quelques tours gratuits supplementaires seraient bien venus. Dans l’ensemble, Spinit Casino offre une experience memorable pour les joueurs en quete d’excitation ! En bonus le design est moderne et dynamique, donne envie de prolonger l’aventure. Particulierement interessant les tournois reguliers pour la competition, renforce le sentiment de communaute.
https://spinitcasinologinfr.com/|
J’ai une passion cyclonique pour Spinit Casino, on ressent une ambiance de piste. La selection de jeux est dynamique, comprenant des jeux compatibles avec les cryptos. 100% jusqu’a 500 € + tours gratuits. Le support client est veloce, toujours pret a accelerer. Les gains arrivent sans delai, de temps a autre plus de promos regulieres ajouteraient du rythme. Dans l’ensemble, Spinit Casino offre une experience memorable pour les joueurs en quete d’excitation ! De plus l’interface est fluide comme une piste, ajoute une touche de vitesse. Egalement appreciable les tournois reguliers pour la competition, propose des avantages personnalises.
casinospinitfr.com|
J’ai une passion mythique pour Olympe Casino, il procure une experience legendaire. Il y a une profusion de jeux captivants, avec des slots thematiques antiques. Le bonus de bienvenue est divin. Le support client est olympien, joignable a toute heure. Les transactions sont fiables, bien que des recompenses supplementaires seraient eternelles. Pour conclure, Olympe Casino est une plateforme qui regne sur l’Olympe pour les passionnes de jeux antiques ! Ajoutons que l’interface est fluide comme un nectar, ajoute une touche de mythologie. Egalement remarquable les options de paris sportifs variees, assure des transactions fiables.
https://olympefr.com/|
J’adore l’atmosphere blues de BassBet Casino, il procure une experience rythmee. Les options sont vastes comme un album, comprenant des jeux compatibles avec les cryptos. Le bonus de bienvenue est rythme. Le support est irreprochable, avec une aide precise. Les paiements sont securises et rapides, de temps a autre des offres plus genereuses seraient blues. En bref, BassBet Casino offre une experience memorable pour ceux qui aiment parier en crypto ! A noter le design est moderne et blues, donne envie de prolonger l’aventure. Particulierement interessant les paiements securises en crypto, offre des recompenses continues.
bassbetcasinologinfr.com|
https://xn--kr41-rzb.com android
trustedpartnergroup – The mobile version of the site is responsive, which is great for on-the-go reference.
ultimateprofitplan – Layout is clean and user-friendly, navigation was smooth for first look.
visionaryfutureteam – Browsing on mobile was smooth, which is always a plus for quick access.
dailyessentialstore – Shipping cost was reasonable and packaging was secure—no damage upon arrival.
trustbridgealliance – Found the platform today, looks like a promising network for professional growth.
سلام خوانندگان، راجع به سایتهای شرطبندی تصور نمیکنید؛
آنها پلتفرمها آکنده از خطرات اقتصادی، روانی
و خانوادگی هستند. من از تک کلیک سرمایهام را باخت نمودم.
سوءاستفاده نسبت به چنین بازیها سریعتر از آن که تصور میکنید رشد مینماید.
به هیچ وجه درگیر نخواهید شد!
professionalgrowthhub – Great resource for advancing careers, lots of practical advice and tools.
1091m2love – Everything loads quickly and looks elegant, awesome presentation all around.
shopandshine – The product I received matched what I saw online, good accuracy.
займ без карты срочно https://zaimy-91.ru
creativefashionworld – The layout is clean and browsing new arrivals was super smooth.
mcalpineinfo – Overall a basic web presence so far; good first step, but needs expansion and verification.
Скачать видео с YouTube https://www.fsaved.com онлайн: MP4/WEBM/3GP, качество 144p–4K, конвертация в MP3/M4A, поддержка Shorts и плейлистов, субтитры и обложки. Без регистрации, быстро и безопасно, на телефоне и ПК. Используйте только с разрешения правообладателя и в рамках правил YouTube.
saveshelterpets – The website design is decent and mobile-friendly, which is positive for user trust.
pmajoe4council – Clear campaign focus, design feels trustworthy and community-oriented.
updatingparents – The site feels thoughtful, user-friendly and clearly grounded in practical support.
Great post however I was wanting to know if you could write a litte more on this topic? I’d be very thankful if you could elaborate a little bit more. Many thanks!
регистрация leebet
Надёжный партнёр для бизнеса, всё предсказуемо и чётко, https://tamozhenniiy-broker-moskva.ru/
Howdy are using WordPress for your site platform? I’m new to the blog world but I’m trying to get started and create my own. Do you need any html coding expertise to make your own blog? Any help would be greatly appreciated!
https://sravni-bank.ru/
Левандовски футболдо чыдамкайлык менен мыкты натыйжа көрсөттү.
Левандовскинин жашоо образы көптөр үчүн мотивация. Левандовски канча жыл ойногонуна карабай, деңгээлин түшүргөн жок. Роберт Левандовски көп жолу “алтын бутсага” татыктуу болгон. Акыркы маектер жана жаңылыктар [url=https://robert-lewandowski-kg.com/]https://robert-lewandowski-kg.com/ сайтында. Роберт Левандовски Барселонада мыкты старт жасаган.
Левандовскинин биографиясы шыктануу жаратат. Левандовскинин булчуңдары жана абалы көпчүлүккө үлгү.
Левандовскинин түпкү теги тууралуу маалымат көп талкууланат. Роберт Левандовски карьерасын толук берилип өткөрөт.
buchmacher bonus
Also visit my blog post ecopayz Wettanbieter
Hi, its fastidious article regarding media print, we all know media is a wonderful source of data.
rdrc.kz
united kingdom pokies online real money, united statesn online real money poker sites and
big usa slot wins, or cahuilla casino ausaa
california
Feel free to visit my web blog – snake eyes in craps (ludoking.com.in)
Explore Vivalis, the perfect place for high-quality
wellness products. Whether you’re enhance your health, we offer a variety of options.
Shop now for the best products and begin your journey towards a healthier lifestyle!
To know more click here:- https://buy.vivalis-usa.com/
What i don’t realize is actually how you’re now not actually much more neatly-liked than you might be now.
You’re so intelligent. You know therefore considerably in the case of this
matter, produced me individually believe it from numerous various angles.
Its like men and women aren’t interested until
it is one thing to do with Woman gaga! Your own stuffs great.
Always care for it up!
My page – Suquamish Indian Casino
Роберт Левандовски футбол дүйнөсүндөгү эң таасирдүү чабуулчулардын бири.
Левандовскинин жашоо образы көптөр үчүн мотивация. Роберт Левандовски жашоосунда көп ийгиликке жеткен. Левандовски Польшанын эң мыкты чабуулчусу катары таанылган. Эгер сиз Левандовскинин биографиясын издеп жатсаңыз, [url=http://robert-lewandowski-kg.com/]http://robert-lewandowski-kg.com/ шилтемесин колдонуңуз. Барселонадагы Левандовскинин оюнун күйөрмандар макташат.
Левандовски футболго болгон сүйүүсүн эч качан жоготпойт. Левандовски спорттук формасын жоготпойт.
Левандовскинин түпкү теги тууралуу маалымат көп талкууланат. Роберт Левандовскинин профессионалдуулугу суктанарлык.
Great insights on MindVault! It’s interesting to see how it enhances focus and memory.
I’ve noticed a significant difference since I started using it!
What tips do you have for maximizing its effects?To know more at buy.mindvault-usa.com!
I believe that SugarMute offers such innovative solutions for sugar cravings!
I’ve seen many great results about their products. Thanks for sharing this information!
For more details, check out https://buy.sugarmute–usa.com/.
Роберт Левандовски акыркы оюндарында мыкты оюн көрсөттү.
Левандовски жубайы менен көп кайрымдуулук иштерин колдойт. Левандовскинин трансфери Барселона үчүн чоң ийгилик болгон. Левандовски Польша командасынын негизги оюнчусу болуп саналат. Барселона, Польша жана Роберт Левандовски тууралуу баарын [url=https://robert-lewandowski-kg.com/]https://robert-lewandowski-kg.com/ сайтынан билиңиз. Барселона жана Левандовски – мыкты тандем.
Роберт Левандовски жашоосунда кыйынчылыктарды жеңип чыккан. Левандовскинин булчуңдары жана абалы көпчүлүккө үлгү.
Роберт Левандовскинин үй-бүлөлүк тамыры Польшада. Роберт Левандовски өзүнүн максатына ишеним менен жеткен.
I can’t believe the improvement I’ve seen with BrainSong!
The audio programs are a game-changer for my memory skills.
Has anyone else had similar experiences? Check out their offerings at buy.brainsong-usa.us!
Роберт Левандовски тууралуу жаңылыктар ар дайым көңүлдү бурат.
Роберт Левандовскинин үй-бүлөлүк жашоосу кызыктуу. Роберт Левандовски жашоосунда көп ийгиликке жеткен. Левандовски футбол тарыхында өз изин калтырган. Левандовскинин карьерасын терең изилдөө үчүн [url=robert-lewandowski-kg.com]robert-lewandowski-kg.com сайты пайдалуу болот. Роберт Левандовски Барселона үчүн көп гол салууда.
Левандовски спорттук тарбиянын маанисин ар дайым белгилейт. Роберт Левандовски реабилитацияны тез бүтүргөн.
Левандовскинин балдары да спортко жакын. Левандовски ар дайым жогорку деңгээлди кармайт.
Great insights on NitricBoost! It’s fascinating to see how it enhances performance.
I’ve experienced significant gains since I started using it!
What tips do you have for maximizing its effects? Check it out at buy.nitricboost–usa.us.
Роберт Левандовскинин машыгуусу көптөргө үлгү.
Левандовскинин жубайы жана үй-бүлөсү тууралуу көптөр кызыгат. Левандовски канча гол салганы тууралуу күйөрмандар ар дайым талашат. Левандовскинин машыгуу режими көпчүлүккө кызыктуу. Кызыктуу фактылар менен таанышуу үчүн [url=http://robert-lewandowski-kg.com/]robert-lewandowski-kg.com[/url] сайтына кириңиз. Бул жерде анын карьерасы тууралуу кеңири маалымат бар. Роберт Левандовски Барселона үчүн көп гол салууда.
Роберт Левандовски карьерасын үзүрлүү өткөрүүдө. Левандовски ден соолугуна кылдат карайт.
Роберт Левандовски үй-бүлөсүнө чоң маани берет. Левандовски ар бир оюнда өзүн көрсөтүүгө аракет кылат.
futuregoalsnetwork.shop – Fast shipping and quality items; very satisfied with my purchase.
Cocaine comes from coca leaves found in South America.
While once utilized in well-known pharmaceutical, it’s instanter a
banned significance due to its dangers. It’s hugely addictive, unsurpassed to healthiness risks like heart attacks, mental disorders, and glowering addiction.
Appreciate this post. Will try it out.
learnsharegrow.shop – The layout is clean and easy to navigate; very user-friendly.
где можно взять займ деньги онлайн займ
займ онлайн срочно где можно взять займ
заем займ онлайн бесплатно
http://buy.mitolyn-sales.us
leadersunitedgroup.bond – The community feedback makes the journey feel shared, not lonely.
globalpartnershipteam.shop – Frequent discounts and promotions; always a great deal to find.
teamfuture.bond – Overall a polished site with professional and engaging content.
discovervalue.bond – Appreciate the practical advice shared; feels grounded and real.
winmore.bond – Impressive visuals and smooth interface; makes browsing enjoyable.
connectinnovationhub.bond – Content is clear, informative, and engaging; great first impression.
trustandunity.bond – Content is clear, informative, and engaging; great first impression.
https://buy.boostaro.co.uk/ + Boostaro
businessgrowthhub.shop – Navigation is intuitive, finding information is quick and effortless.
smartfashionboutique.bond – Overall a polished site with professional and engaging content.
successfultradersclub.shop – Typography and design are consistent and visually appealing.
globalalliancenetwork.bond – Fast loading pages make the experience seamless and smooth.
Explore Prostavive USA, your ultimate destination for wellness solutions.
If you’re looking to enhance your health You’ve come to
the right place! Check out our bestsellers today!
uniquedecorstore.shop – Content is clear, informative, and engaging; great first impression.
Je suis epate par BassBet Casino, c’est une plateforme qui vibre comme un festival. Le catalogue est riche en surprises, incluant des paris sportifs pleins de vie. Amplifiant l’excitation du jeu. Disponible 24/7 via chat ou email, avec une aide precise. Les transactions sont fiables, parfois des bonus plus varies seraient un hit. Pour conclure, BassBet Casino est un must pour les joueurs pour les fans de casino en ligne ! De plus l’interface est fluide comme un mix, donne envie de prolonger la fete. Egalement genial les tournois reguliers pour la competition, offre des recompenses continues.
https://bassbetcasinoappfr.com/|
J’ai un faible eclatant pour BassBet Casino, ca transporte dans un univers de lumieres. Le catalogue est riche en surprises, avec des slots aux designs fluorescents. Amplifiant l’excitation du jeu. Disponible 24/7 via chat ou email, offrant des solutions claires. Les gains arrivent sans attendre, de temps en temps plus de promos regulieres ajouteraient du scintillement. Pour conclure, BassBet Casino est un must pour les joueurs pour les fans de casino en ligne ! Ajoutons que l’interface est fluide comme un neon, booste le plaisir de jouer. A souligner les tournois reguliers pour la competition, qui dynamise l’engagement.
bassbetcasinopromocodefr.com|
Je suis emerveille par BassBet Casino, ca offre un plaisir nautique. Le catalogue est riche et varie, proposant des jeux de table elegants. Le bonus de bienvenue est rapide. Les agents repondent comme une vague, garantissant un support de qualite. Les transactions sont fiables, neanmoins plus de promos regulieres ajouteraient du rythme. Dans l’ensemble, BassBet Casino garantit un plaisir constant pour les joueurs en quete d’excitation ! En bonus l’interface est fluide comme un lac, amplifie le plaisir de jouer. Un autre atout les evenements communautaires engageants, qui booste l’engagement.
bassbetcasinobonusfr.com|
J’ai une passion parfumee pour Spinit Casino, on ressent une ambiance delicate. Il y a une profusion de jeux captivants, offrant des sessions live sophistiquees. Amplifiant le plaisir de jeu. L’assistance est efficace et professionnelle, joignable a toute heure. Les retraits sont fluides comme un parfum, bien que plus de promos regulieres ajouteraient du piquant. Pour conclure, Spinit Casino offre une experience memorable pour ceux qui aiment parier en crypto ! A noter la navigation est simple et gracieuse, ajoute une touche d’elegance. Un plus les paiements securises en crypto, offre des recompenses continues.
spinitcasinobonusfr.com|
J’ai une affection pour Impressario Casino, on ressent une ambiance delicate. Le catalogue est riche en saveurs, incluant des paris sportifs distingues. Le bonus de bienvenue est delicieux. Les agents repondent avec courtoisie, offrant des reponses claires. Les retraits sont fluides comme la Seine, neanmoins des recompenses additionnelles seraient royales. Pour conclure, Impressario Casino vaut une visite sophistiquee pour les amateurs de sensations elegantes ! A noter la plateforme est visuellement somptueuse, ce qui rend chaque session plus raffinee. A souligner le programme VIP avec niveaux exclusifs, renforce le sentiment de communaute.
Avancer|
http://gallery2.papa-power.com/view.ppw?b_idx=99998738
J’aime l’ambiance crypto de Monte Cryptos Casino, ca transporte dans un desert futuriste. Les options sont vastes comme un horizon, proposant des jeux de table sophistiques. 100% jusqu’a 300 € + tours gratuits. Le suivi est d’une efficacite absolue, joignable a tout moment. Les transferts sont fiables, parfois des bonus plus varies seraient un mirage. Au final, Monte Cryptos Casino est un must pour les fans de blockchain pour ceux qui parient avec des cryptos ! Par ailleurs l’interface est fluide comme un vent du desert, facilite une immersion totale. Egalement cool les paiements securises en BTC/ETH, propose des avantages uniques.
Visiter la page web|
J’adore l’elegance de Impressario Casino, ca offre un plaisir sophistique. Le catalogue est riche en saveurs, avec des slots aux designs elegants. Avec des depots instantanes. Le support client est impeccable, garantissant un support de qualite. Les transactions sont fiables, cependant des offres plus genereuses seraient exquises. En resume, Impressario Casino vaut une visite sophistiquee pour ceux qui aiment parier en crypto ! A noter le design est moderne et chic, ajoute une touche d’elegance. Particulierement interessant les tournois reguliers pour la competition, offre des recompenses continues.
Trouver la vГ©ritГ©|
I really enjoyed this article. Thanks for sharing your thoughts on this
topic. I found it particularly interesting how you discussed the role
of technology in today’s world. For more information, check out(https://buy.arialief–usa.us/).
nextvision.bond – The content is engaging and thought-provoking; keeps me coming back.
Ледипасвир Вирус гепатита C передается преимущественно парентеральным путем, то есть через кровь. Основные факторы риска включают использование нестерильных медицинских инструментов, переливание инфицированной крови (хотя в настоящее время это встречается крайне редко благодаря скринингу донорской крови), употребление инъекционных наркотиков (особенно при совместном использовании игл), нанесение татуировок или пирсинга в ненадлежащих условиях. Вертикальная передача от матери к ребенку во время беременности или родов также возможна, но вероятность относительно низкая.
successpathway.bond – Impressed with the product quality and packaging; exceeded expectations.
Welcome to the latest in wellness products at the Neuroxen store.
If you are looking for supplements, this site provides a variety of choices to support your lifestyle.
Take a look at the top-rated products that have received great feedback.
Join the community! Visit [buy.neuroxen-usa.com](https://buy.neuroxen-usa.com) for special discounts.
growthpoint.bond – Impressive design and user experience; feels professional and welcoming.
Thank you for any other wonderful article. The place else may anyone get that type of information in such an ideal way of writing? I have a presentation next week, and I am at the look for such info.
купить виртуальный номер
прокси с ротацией Мобильные прокси – это специализированный тип прокси-серверов, использующих IP-адреса, присвоенные мобильным операторам. Это делает их особенно ценными для задач, требующих высокой степени анонимности и избежания блокировок, поскольку эти IP-адреса ассоциируются с реальными мобильными устройствами. В отличие от обычных прокси, мобильные прокси сложнее заблокировать, так как это может привести к блокировке широкого диапазона IP-адресов, используемых легитимными пользователями.
экскурсии из шарджи Туры в ОАЭ – это увлекательное путешествие по семи эмиратам, каждый из которых обладает своим неповторимым характером и шармом. Откройте для себя разнообразие ландшафтов, богатство культуры и великолепие архитектуры этой удивительной страны. Шарджа достопримечательности, экскурсии из шарджи и отдых в фуджейре дополнят вашу палитру путешествий по ОАЭ.
createimpacttoday.shop – Fast shipping and excellent customer service; will shop here again.
uniquefashionstyle.shop – Really like the vibe here—feels like a boutique you’d walk into.
play-brary.org – Navigation is smooth and intuitive, making browsing enjoyable.
discovergreatdeal.shop – Wide range of categories; something for everyone here.
leanneslifechangingfairies.com – The site looks friendly and welcoming, very easy to use.
https://dzen.ru/a/aPRXjPraMEja6Ols При выборе виртуального номера важно учитывать ряд факторов, таких как надежность провайдера, доступность функций и стоимость услуг. Надежный провайдер обеспечит стабильную работу виртуального номера и конфиденциальность переписки. Доступность функций, таких как переадресация SMS, голосовая почта и интеграция с другими сервисами, позволит максимально эффективно использовать виртуальный номер. Стоимость услуг должна быть разумной и соответствовать потребностям пользователя. Купить виртуальный номер – это инвестиция в свою безопасность и комфорт в цифровом мире.
I found a lot of value in this article, thank you for sharing.
If anyone is interested, I also wrote about this topic here:
https://buy.lavaslim–usa.com/.
Excellent read! It helped me clarify a few doubts I had.
For more details, you can check: https://buy.lavaslim–usa.com/.
Great post! Your perspective is refreshing.
The way you explained the importance of innovation in modern society.
You might also like to explore [Completethyroid](https://buy.info-completethyroid.us/)
futureopportunitynetwork.shop – Customer service was helpful and responsive; will shop again.
1xbet turkey [url=https://1xbet-10.com]https://1xbet-10.com[/url] .
1xbet g?ncel giri? [url=www.1xbet-14.com]1xbet g?ncel giri?[/url] .
кухни на заказ в спб недорого [url=kuhni-spb-2.ru]kuhni-spb-2.ru[/url] .
discovernewpath.shop – Love the clean design and easy navigation; feels very welcoming.
I think that AquaSculpt USA offers such advanced body sculpting solutions!
I’ve seen many good reviews about their techniques.
I’m impressed by how the team addresses trouble spots.
I can’t wait to learn more! I appreciate the insights!
For more details, check out https://buy.aquasculpt–usa.com/.
Роберт Левандовски футбол дүйнөсүндөгү эң таасирдүү чабуулчулардын бири.
Левандовскинин жашоо образы көптөр үчүн мотивация. Левандовски канча гол салганы тууралуу күйөрмандар ар дайым талашат. Левандовски Польша командасынын негизги оюнчусу болуп саналат. Футбол күйөрмандары үчүн мыкты булак — [url=robert-lewandowski-kg.com]robert-lewandowski-kg.com</url]. Бул сайтта акыркы жаңылыктар топтолгон. Барселона жана Левандовски – мыкты тандем.
Левандовски футболго болгон сүйүүсүн эч качан жоготпойт. Роберт Левандовскинин жаракаты көпчүлүктү тынчсыздандырган.
Роберт Левандовскинин чоң атасы тууралуу уламыштар айтылат. Левандовски ийгиликке жөн эле жеткен эмес.
Ich bin fasziniert von Snatch Casino, es bietet ein immersives Erlebnis. Es gibt unzahlige packende Spiele, mit aufregenden Live-Casino-Erlebnissen. Mit schnellen Einzahlungen. Die Mitarbeiter sind schnell und kompetent. Transaktionen sind immer sicher, gelegentlich zusatzliche Freispiele waren willkommen. Insgesamt, Snatch Casino ist ein Ort fur pure Unterhaltung. Zusatzlich ist das Design zeitgema? und attraktiv, zum Weiterspielen animiert. Ein bemerkenswertes Feature die regelma?igen Turniere fur Wettbewerbsspa?, individuelle Vorteile liefern.
snatch-casino.de|
mdcphilly.com – Navigation feels intuitive; I found what I needed quickly without confusion.
harrietlevinmillan.com – Looking forward to following updates and new writings here.
ks4thekids.com – Feels professional yet accessible; nice balance overall.
I believe that slimjaro usa offers such advanced body
sculpting solutions! I’ve seen many good reviews about their services.
I’m impressed by how the team addresses stubborn fat areas.
I’m looking forward to trying it out! I appreciate the
insights!
For more details, check out https://buy.slimjaro–usa.us/.
brauhausschmitzoktoberfest.com – The information is clear and upfront—nice layout.
pp4fdr.org – Browsing this site was easy and I found the content very well-laid-out.
clubinorout.com – Looking forward to seeing how this site evolves and what’s added.
My issues have been very similar, with my family. But, we made some different decisions. It’s complex.
concernedaboutpollution.com – I found the content informative and easy to follow; good job.
mdcphilly.com – The typography and spacing really enhance readability; good choice.
asianspeedd8.com – The navigation is intuitive; finding information was straightforward.
berrybombselfiespot.com – Looks like a fun outing for friends, content creators, and social media lovers.
komunahouse.com – The visuals really reflect the artistic mission—nice aesthetic.
grant-jt.com – The site’s design feels clean and minimal, nice first impression.
gailcooperspeaker.com – I found the content genuine and relatable; good job.
reindeermagicandmiracles.com – The tone is cheerful and inviting; makes me smile.
brucknerbythebridge.com – The site gives off a modern and urban-chic vibe; nice first impression.
meetkatemarshall.com – The design is clean and professional, yet personal enough to feel authentic.
Wish I’d thought of this. Am in the field, but I procrastinate alot and haven’t written as much as I’d like. Thanks.
moeinclub.com – It’s helpful that recent posts are clearly visible — keeps things fresh.
shemplymade.com – I like the handcrafted vibe; it makes the brand feel personal.
centensports.com – Navigation feels intuitive and finding key sections is straightforward.
” />safercharging.com – (Note: I saw a small URL redirect oddness when checking a link.)
seenministries.com – I like the emphasis on community and connection; it feels inclusive.
opensky-inc.com – The design is professional yet approachable — good balance.
pastorjorgetrujillo.com – Looking forward to future updates and seeing how the website evolves.
casa-nana.net – Looks like a reliable organization; the presentation builds confidence.
trendingnewsfeed.net – content appears varied, which keeps things interesting.
Авто под заказ Японии Выездная диагностика Магнитогорск – это удобный сервис, позволяющий оценить состояние автомобиля непосредственно на месте его нахождения. Наши эксперты проведут комплексную проверку кузова, двигателя, трансмиссии, подвески и электроники, используя современное диагностическое оборудование. Мы предоставим вам подробный отчет о состоянии автомобиля, выявив все имеющиеся дефекты и рекомендовав необходимые ремонтные работы.
clevelandchristmasclub.com – Would love to see more detailed event schedules and pricing info.
electmikemontes.com – Navigation is intuitive and the key information is easy to find.
دوستان گرامی، سایتهای
شرطبندی نه فقط سرمایهتان را ربایش میکنند، همچنین ذهن جسمیتان را تخریب مینمایند.
من فعالیت با هیجان شدم، اما فوری مبتلا سوءمصرف گردید و کارام
ترک شد. خطر چنین جاها عمیقتر از امری است که ذهن میکنید.
اجتناب کامل به این لازم است!
Земельные участки Документы недвижимости: экспертный анализ и подготовка.
kraken ссылка Kraken market – это многогранная площадка, где встречаются продавцы и покупатели со всего мира, предлагая широкий спектр товаров и услуг. Здесь можно найти все, что душе угодно, от редких артефактов до инновационных разработок. Приготовьтесь к захватывающему приключению, полному неожиданных открытий и выгодных предложений. Но не забывайте о здравом смысле и критическом мышлении, чтобы не стать жертвой недобросовестных продавцов.
It’s an amazing piece of writing for all the internet viewers; they will get benefit from
it I am sure.
BassBet Casino m’a ensorcele BassBet Casino, on ressent une energie vibrante. Les titres sont d’une richesse envoutante, avec des slots aux designs soul. Avec des depots ultra-rapides. Le support client est au top, joignable a tout moment. Les transactions sont fiables, neanmoins plus de promos regulieres ajouteraient du rythme. Pour conclure, BassBet Casino offre une experience inoubliable pour les passionnes de jeux modernes ! Ajoutons que l’interface est fluide comme une chanson, facilite une immersion totale. Particulierement cool le programme VIP avec des niveaux exclusifs, offre des recompenses continues.
https://bassbetcasinopromocodefr.com/|
Je suis emerveille par Spinit Casino, on ressent une ambiance de fees. Il y a une profusion de jeux fascinants, avec des slots aux designs enchantes. Amplifiant le plaisir de jeu. Le service est disponible 24/7, joignable a toute heure. Les transactions sont fiables, neanmoins des offres plus genereuses seraient enchantees. En resume, Spinit Casino offre une experience memorable pour les amateurs de sensations enchantees ! Ajoutons que l’interface est fluide comme un conte, ce qui rend chaque session plus enchantee. Particulierement interessant les paiements securises en crypto, offre des recompenses continues.
spinitcasinobonusfr.com|
J’adore l’atmosphere musicale de BassBet Casino, ca plonge dans un monde de notes. Il y a une profusion de jeux excitants, avec des slots aux designs rythmiques. Le bonus de bienvenue est rythme. Le support est irreprochable, avec une aide precise. Le processus est simple et elegant, de temps a autre quelques tours gratuits supplementaires seraient bien venus. En bref, BassBet Casino vaut une jam session pour les passionnes de jeux modernes ! Par ailleurs la navigation est simple et rythmee, donne envie de prolonger l’aventure. Particulierement interessant les tournois reguliers pour la competition, assure des transactions fiables.
https://bassbetcasinobonusfr.com/|
gratiswette ohne einzahlung sofort
Feel free to surf to my page: beste Sportwetten tipps
Je suis accro a Monte Cryptos Casino, il procure une aventure virtuelle. Les options sont vastes comme un reseau, avec des slots aux themes innovants. Le bonus d’accueil est eclatant. Les agents repondent avec une rapidite cosmique, offrant des reponses claires. Le processus est lisse comme un wallet, neanmoins des offres plus genereuses ajouteraient du charme. En resume, Monte Cryptos Casino garantit un plaisir constant pour les adeptes de jeux modernes ! Ajoutons que le design est moderne et captivant, facilite une immersion totale. A souligner les options de paris variees, offre des recompenses continues.
Visiter maintenant|
Je suis seduit par Impressario Casino, il procure une experience exquise. La selection de jeux est somptueuse, avec des slots aux designs elegants. Amplifiant le plaisir de jeu. L’assistance est efficace et professionnelle, garantissant un support de qualite. Les gains arrivent sans delai, cependant quelques tours gratuits supplementaires seraient bien venus. Au final, Impressario Casino garantit un plaisir constant pour les fans de casino en ligne ! Par ailleurs le site est rapide et attractif, donne envie de prolonger l’experience. Particulierement interessant le programme VIP avec niveaux exclusifs, offre des recompenses continues.
Obtenir l’offre|
Je suis absolument ravi par Impressario Casino, ca transporte dans un monde chic. La selection de jeux est exquise, comprenant des jeux compatibles avec les cryptos. Avec des depots instantanes. Le suivi est irreprochable, offrant des reponses claires. Les transactions sont fiables, cependant des bonus plus varies seraient un plus. Dans l’ensemble, Impressario Casino garantit un plaisir constant pour les passionnes de jeux modernes ! A noter la navigation est simple et gracieuse, donne envie de prolonger l’experience. Un autre atout les options de paris sportifs variees, qui booste l’engagement.
Commencer l’aventure|
1xbet mobi [url=http://1xbet-17.com/]1xbet mobi[/url] .
dankglassonline.com – It might benefit from more descriptive about-pages to build trust.
J’ai une affection pour Impressario Casino, ca transporte dans un monde gourmand. Les options sont vastes comme un menu etoile, comprenant des jeux compatibles avec les cryptos. Avec des depots instantanes. Le support client est impeccable, joignable a toute heure. Les paiements sont securises et rapides, de temps a autre des offres plus genereuses seraient exquises. Pour conclure, Impressario Casino est une plateforme qui enchante pour les passionnes de jeux modernes ! Par ailleurs la plateforme est visuellement somptueuse, ajoute une touche d’elegance. Un plus les evenements communautaires engageants, assure des transactions fiables.
Lire les dГ©tails|
медицинское оборудование для больниц [url=www.medicinskoe–oborudovanie.ru]www.medicinskoe–oborudovanie.ru[/url] .
Pretty! This has been an incredibly wonderful post. Thank you for supplying this info.
https://kryazh.com.ua/polnoe-rukovodstvo-po-ukhodu-za-kolpakom-dlya-kureniya/
sportwetten anbieter ohne deutsche lizenz
My site; pferderennen wetten regeln (Gustavo)
Does it look like we’re in for a big ride here?
наркологическая клиника трезвый выбор [url=https://narkologicheskaya-klinika-24.ru/]narkologicheskaya-klinika-24.ru[/url] .
Hi there to all, because I am really eager of reading this weblog’s post to be updated on a regular basis. It consists of pleasant material.
https://lummi.com.ua/yak-kupyty-sklo-far-dlya-avto-z-harantiyeyu-ta-bez.html
catherinewburton.com – I’d love to see more testimonials or client stories to deepen connection.
vsmphotography.com – The branding feels consistent and professional throughout the site.
exploreopportunitiesnow.shop – (Note: I noticed a double-URL prefix when checking—a small fix may help.)
What’s up to every body, it’s my first pay a visit of this web site; this web site contains remarkable and truly fine information designed for visitors.
https://diamond-gallery.in.ua/iak-vybraty-dekoratyvni-masky-dlia-sportyvnykh-avto.html
toddstarnesbooktour.com – The site is very clean and organized, making it easy to understand the book tour details.
createandgrow.shop – I like the minimalist design approach; it helps focus on the content.
die besten wetten
My website; online-wetten
startsomethinggreat.shop – The site loads quite fast; browsing feels smooth on mobile too.
findwhatyoulove.shop – I’ll bookmark this to check back for new items or deals.
theperfectgiftshop.com – I like the consistent branding and the inviting design.
shopandsmilealways.shop – It might benefit from more detailed product information to build trust.
Amazing! Your site has quite a few comment posts. How did you get all of these bloggers to look at your site I’m envious! I’m still studying all about posting articles on the net. I’m going to view pages on your website to get a better understanding how to attract more people. Thank you!
simplybestchoice.shop – The minimal design helps focus on the product without distraction.
keepgrowingforward.shop – The layout is clean and simple; very easy to use.
shopandsmilealways.shop – I like how the key message “Smile Always” comes through—it feels positive.
shopandshineeveryday.shop – I like how the key message “Shine Everyday” comes through—it feels positive.
ламинат Магазин напольных покрытий – это ваш надежный проводник в мире полов. Здесь представлен широкий ассортимент материалов от ведущих производителей, способных удовлетворить любые запросы и бюджеты. Квалифицированные консультанты помогут вам подобрать оптимальное решение, учитывая особенности помещения, ваши личные предпочтения и финансовые возможности. Помимо самих покрытий, в магазине вы найдете все необходимые сопутствующие товары: подложки, плинтусы, клеи, герметики и средства для ухода за полом. Ваша задача – лишь выбрать, а наша – предоставить все необходимое для создания идеального пола в вашем доме.
geteventclipboard.com – The layout is professional and straightforward; good first impression.
Найдите лучший товар на https://n-katalog.ru: подробные карточки, честные отзывы, рейтинги, фото, фильтры по параметрам и брендам. Сравнение цен, акции, кэшбэк-предложения и графики изменений. Примите уверенное решение и переходите к покупке в удобном магазине.
микрозайм онлайн онлайн займ
En el sitio https://fotoredaktor.top se indicaba claramente la superficie de Luxemburgo.
uniquetrendstore – Excellent selection of products, always find what I need here.
wetten heute
Feel free to surf to my web blog: sportwetten strategie
newseasoncollection – Fantastic variety of products, always something new and exciting.
smartchoiceoutlet – Excellent selection of products, always find what I need here.
joinourcreativeworld – Fantastic variety of products, always something new and exciting.
inspireeverydaylife – Easy to navigate, found exactly what I was looking for.
shopwithconfidence – Customer support helped me instantly, very impressed with service quality.
dreamcreateinspire – Excellent customer service, resolved my issue quickly and efficiently.
shopthelatesttrend – Excellent selection of products, always find what I need here.
die besten Schleswig Holstein Sportwetten Lizenz
shopforhappiness – Great prices and quality, will definitely shop here again.
shopthebesttoday – Love the unique finds here, always something special to discover.
discovernewworld – Great prices and quality, will definitely shop here again.
BassBet Casino m’a ensorcele BassBet Casino, ca offre un plaisir melodique. Les titres sont d’une richesse envoutante, avec des slots aux designs soul. Avec des depots ultra-rapides. L’assistance est rapide et pro, toujours pret a chanter. Le processus est simple et vibrant, neanmoins des offres plus genereuses seraient soul. Dans l’ensemble, BassBet Casino est une plateforme qui groove pour les amateurs de sensations vibrantes ! A noter le design est moderne et soul, donne envie de prolonger la melodie. Un point fort les tournois reguliers pour la competition, propose des avantages sur mesure.
https://bassbetcasinopromocodefr.com/|
J’adore l’atmosphere dynamique de Spinit Casino, ca plonge dans un monde de vitesse. Il y a une profusion de jeux excitants, comprenant des jeux compatibles avec les cryptos. Amplifiant le plaisir de jeu. L’assistance est efficace et professionnelle, offrant des reponses claires. Les transactions sont fiables, cependant quelques tours gratuits supplementaires seraient bien venus. Pour conclure, Spinit Casino offre une experience memorable pour les fans de casino en ligne ! Ajoutons que la plateforme est visuellement veloce, ajoute une touche de vitesse. Particulierement interessant les options de paris sportifs variees, qui booste l’engagement.
casinospinitfr.com|
globalmarketplacehub – Fantastic variety of products, always something new and exciting.
discoverendlessideas – A go-to place for creative ideas and insightful articles.
J’ai une passion rythmique pour BassBet Casino, on ressent une ambiance de studio. La variete des titres est harmonieuse, proposant des jeux de table elegants. Amplifiant le plaisir de jeu. Le support est irreprochable, joignable a toute heure. Les retraits sont fluides comme un riff, cependant quelques tours gratuits supplementaires seraient bien venus. Dans l’ensemble, BassBet Casino garantit un plaisir constant pour les amateurs de sensations rythmees ! En bonus la navigation est simple et rythmee, ce qui rend chaque session plus groovy. Un autre atout les paiements securises en crypto, renforce le sentiment de communaute.
bassbetcasinobonusfr.com|
everytrendinone – The writing style is clear and friendly—makes reading fun rather than a chore.
Je suis emerveille par Spinit Casino, on ressent une ambiance de fees. Les options sont vastes comme un grimoire, proposant des jeux de table elegants. Avec des depots instantanes. Le support client est enchante, avec une aide precise. Les paiements sont securises et rapides, neanmoins des bonus plus varies seraient un chapitre. Pour conclure, Spinit Casino vaut une lecture magique pour les passionnes de jeux modernes ! Par ailleurs la plateforme est visuellement envoutante, facilite une immersion totale. Particulierement interessant les evenements communautaires engageants, offre des recompenses continues.
spinitcasinobonusfr.com|
brightfuturedeals – The website layout is clean and navigation feels very intuitive today.
Je suis ebloui par Monte Cryptos Casino, il propose une odyssee chiffree. Le catalogue est opulent et divers, proposant des tables sophistiquees. 100% jusqu’a 300 € + tours gratuits. Le suivi est d’une efficacite absolue, toujours pret a decoder. Les paiements sont securises par blockchain, neanmoins des recompenses supplementaires seraient ideales. En bref, Monte Cryptos Casino vaut une exploration virtuelle pour les adeptes de jeux modernes ! A noter la navigation est simple comme un wallet, facilite une immersion totale. Egalement cool les evenements communautaires decentralises, propose des avantages uniques.
Explorer tout le site|
Je suis absolument ebloui par Impressario Casino, il evoque un tapis rouge scintillant. Les titres proposes sont d’une elegance rare, offrant des sessions live immersives. Illuminant l’experience de jeu. Disponible 24/7 via chat ou email, garantissant un service de star. Le processus est simple et gracieux, neanmoins quelques tours gratuits supplementaires seraient apprecies. Dans l’ensemble, Impressario Casino est une plateforme qui captive pour les amateurs de casino en ligne ! En plus la navigation est intuitive et elegante, amplifie le plaisir de jouer. Un atout le programme VIP avec des niveaux exclusifs, propose des avantages sur mesure.
Voir maintenant|
Aw, this was an incredibly nice post. Spending some time and actual effort to generate a superb article… but what can I say… I hesitate a whole lot and never seem to get anything done.
kra42 cc
learnshareconnect – Great quality for the price; items exceeded my expectations easily.
Je suis absolument seduit par Monte Cryptos Casino, ca offre un plaisir numerique unique. Les options sont vastes comme un reseau, incluant des paris en direct dynamiques. 100% jusqu’a 300 € + tours gratuits. Disponible 24/7 via chat ou email, avec une aide precise et rapide. Les retraits sont rapides comme une transaction blockchain, de temps a autre des offres plus genereuses ajouteraient du charme. Pour conclure, Monte Cryptos Casino vaut une exploration virtuelle pour les joueurs en quete d’innovation ! En bonus le design est moderne et captivant, amplifie le plaisir de jouer. Un atout cle les options de paris variees, propose des avantages uniques.
Trouver maintenant|
newthisdomainsssss – I’ll check again later; first impression shows promise but needs definition.
discovergreatthings – Great design and fresh selection, made browsing really fun today.
findyourperfectdeal – Great site that sparks creativity and gives me new perspectives daily.
exploreamazingideas – Great browsing experience, layouts look clean and easy to navigate.
yourjourneybegins – Customer support was helpful and responsive, solved my issue instantly.
brainsight-reeracoen – The interface and communication were so clear, no hidden surprises at all.
blinkiesdonutsca – Staff helped me pick the perfect dozen, thanks for recommendations!
shopwithconfidence – Love how easy it is to find good deals here lately.
Знайшовся на https://siviagmen.com хороший сервіс в Рівному.
Про шифер https://buybuyviamen.com резиновый впервые прочитали в обзоре.
How come you do not have your website viewable in mobile format? cant see anything in my Droid.
Информацию на https://mr-master.com.ua/uk/ использовала для заделки щелей в полу.
tabitoshigoto – Looks like a reliable source to find unique stay-and-work destinations.
realherschel – They answered my query quickly via chat which is a plus.
I just couldn’t depart your website before
suggesting that I actually enjoyed the usual info an individual provide in your visitors?
Is going to be again steadily to investigate cross-check new
posts
ohmydrifter – One suggestion: add some more interactive features like comments or community section.
На сайті zebraschool.com.ua замовив ремонт коляски в Луцьку — зробили на відмінно
Hi, this weekend is nice in support of me, because this moment i am reading this enormous educational paragraph here at my house.
kra42 cc
hopeandlace – Worth bookmarking and exploring their “About” and “Work” sections for deeper understanding.
motivilovesmusic – Might be useful as an additional link, but I’d balance it with higher-authority sources.
https://zumo-spin-games.com/
trabas007hoki – Overall okay for now, but I’d proceed cautiously before making any big commitment.
envolavecning – If you’re considering using them, I’d check who’s behind the domain and look for reviews or trust signals.
hanayaka-na-life – Really enjoy the vibe of the brand, clean aesthetic and easy to navigate.
hawaiineiartcontest – Overall, this looks like a well-organized event with a clear mission and opportunity for exposure.
wrestlingac – If you plan to use it as a reference, check the specific article date and author to ensure timeliness.
sega-live – Navigation is intuitive and items display well, though I’d want to check payment security.
beauty-optical-salon – Located in New York? No, actually in Shinjuku, Tokyo at the LUMINE2 building. :contentReference[oaicite:5]index=5
hopprojects – The location in Folkestone’s Creative Quarter gives it a unique cultural energy.
kraken market Kraken сайт – это таинственный портал в мир эксклюзивных возможностей, где анонимность и безопасность возведены в абсолют. Здесь границы стираются, а мечты обретают реальность, но помните: с большой свободой приходит и большая ответственность. Каждый шаг требует осознанности и понимания, ведь за порогом скрываются не только сокровища, но и подводные камни. Подходите к изучению платформы с мудростью и осторожностью, взвешивая каждый свой выбор.
foruminvestmali – The domain was registered in 2017 and appears to have low traffic and minimal backlink profile. :contentReference[oaicite:0]index=0
joebobsaveschristmas – Love the color scheme and graphics, very distinctive and memorable.
rebeccacaridad-manzanita – The name “Manzanita” might connect to the blog or creative brand of Rebecca Caridad, which could be fine but still needs verification.
codes promo gratuits pour 1xbet Ultimately, the goal is to find a “code promo pour 1xBet” or “code promo sur 1xbet” that provides a real advantage. Whether it’s a “bonus d’inscription 1xbet” or a “code promo 1xbet 131$,” smart bettors understand the power of a well-chosen promotional code. Thorough research, verification, and understanding the terms and conditions are paramount to successfully leveraging these offers.
Howdy! This is my first comment here so I just wanted to give a quick shout out and tell you I genuinely enjoy reading your posts. Can you recommend any other blogs/websites/forums that cover the same subjects? Thank you so much!
kra42 at
globaltrendmarket – Overall: promising concept, all the usual due diligence (contact info, business disclosures) recommended.
dearsparrows – I appreciate the variety of topics covered, from racing to casino trends and more.
pepplish – The “locally-sourced” and “small-batch” language is appealing if authenticity is important to you.
nomnomnomfordogs – Great option if you live nearby or want artisan-style dog treats rather than mass-produced.
connectdiscovergrow – The tagline “Connect. Discover. Grow.” really speaks to networking and growth opportunities.
flourandoak – The branding “Flour & Oak” suggests craftsmanship and wood-fired or rustic baking, which is appealing.
shopwithconfidence – Navigation feels intuitive, but it’s not clear what types of products or services are offered.
вывод из запоя москва клиника [url=https://narkologicheskaya-klinika-25.ru]https://narkologicheskaya-klinika-25.ru[/url] .
brightnightpdx – A suggestion: check domain age and whether the SSL certificate is up to date to ensure it’s safe.
keepgrowingforward – Would like to see a bit more about who’s behind the site for extra trust.
Куда сходить в Питере Обязательно посетите Петропавловскую крепость – сердце города. Поднимитесь на колокольню собора Петра и Павла, откуда открывается захватывающий вид на город, прогуляйтесь по территории крепости, узнайте об истории императорской семьи Романовых.
staycuriousalways – I’d check for contact details and about-page to ensure it’s well maintained and trustable.
theperfectgiftshop – The domain name is appealing and suggests a curated gift-shop vibe right away.
exploreopportunitiesnow – Might be ideal as a secondary resource in a network of references, rather than a primary authority.
bestdealsforlife – I recommend verifying domain age and ownership details for more trust-worthiness.
createandgrow – Navigation is smooth, but I didn’t immediately spot contact info or about details.
Quality posts is the key to invite the users to visit
the web page, that’s what this site is providing.
What’s up friends, pleasant paragraph and good arguments commented here, I am really enjoying by these.
kra41 cc
buildyourdreamtoday – The domain has potential, but more content and clarity are needed to assess its value.
Je suis emerveille par Olympe Casino, ca transporte dans un monde mythique. Le catalogue est riche en melodies, comprenant des jeux optimises pour les cryptos. Amplifiant l’aventure de jeu. Le support client est olympien, avec une aide precise. Les gains arrivent sans delai, cependant des bonus plus varies seraient un nectar. Dans l’ensemble, Olympe Casino est une plateforme qui regne sur l’Olympe pour les amateurs de sensations mythiques ! Par ailleurs la navigation est simple comme un oracle, ce qui rend chaque session plus celeste. Particulierement captivant les evenements communautaires engageants, renforce le sentiment de communaute.
olympefr.com|
wettanbieter deutschland
Here is my site: gratis guthaben Ohne einzahlung Sportwetten
ExploreAmazingIdeas – Always inspired by the content, website makes browsing very enjoyable.
uniquetrendstore – If you are buying from or linking to the site, make sure to review shipping, return policy, and product quality carefully.
InspireEverydayLife – Highly recommend this site, offers valuable content that is genuinely useful.
ShopTheBestToday – Fast delivery, products arrived safely and exactly as described online today.
Ich habe eine Leidenschaft fur Cat Spins Casino, es bietet ein immersives Erlebnis. Es gibt eine enorme Vielfalt an Spielen, mit Live-Sportwetten. Er macht den Start aufregend. Der Kundendienst ist ausgezeichnet. Die Zahlungen sind sicher und sofortig, in manchen Fallen gro?zugigere Angebote waren klasse. Letztlich, Cat Spins Casino bietet ein gro?artiges Erlebnis. Daruber hinaus ist das Design stilvoll und einladend, jeden Moment aufregender macht. Ein tolles Feature die regelma?igen Turniere fur Wettbewerbsspa?, ma?geschneiderte Privilegien liefern.
Jetzt stöbern|
Ich bin absolut begeistert von Cat Spins Casino, es ist ein Ort, der begeistert. Es gibt eine Fulle an aufregenden Titeln, mit Spielen, die Krypto unterstutzen. Der Willkommensbonus ist gro?zugig. Erreichbar rund um die Uhr. Die Zahlungen sind sicher und zuverlassig, dennoch zusatzliche Freispiele waren ein Bonus. Letztlich, Cat Spins Casino ist ein Muss fur Spieler. Nebenbei die Plattform ist visuell ansprechend, eine immersive Erfahrung ermoglicht. Ein besonders cooles Feature die regelma?igen Wettbewerbe fur Spannung, die die Motivation erhohen.
Details prГјfen|
Je suis bluffe par Ruby Slots Casino, on y trouve une vibe envoutante. Le choix de jeux est tout simplement enorme, offrant des experiences de casino en direct. Le bonus de depart est top. Le support est rapide et professionnel. Les retraits sont lisses comme jamais, quelquefois quelques tours gratuits supplementaires seraient cool. Pour conclure, Ruby Slots Casino offre une aventure memorable. A mentionner la navigation est claire et rapide, incite a rester plus longtemps. Un point cle le programme VIP avec des avantages uniques, offre des bonus exclusifs.
Aller Г la page|
Je suis sous le charme de Sugar Casino, ca invite a l’aventure. La bibliotheque de jeux est captivante, proposant des jeux de table sophistiques. Il propulse votre jeu des le debut. Les agents repondent avec efficacite. Les gains arrivent sans delai, malgre tout des offres plus importantes seraient super. En fin de compte, Sugar Casino garantit un amusement continu. De surcroit le design est tendance et accrocheur, booste l’excitation du jeu. A mettre en avant les options de paris sportifs diversifiees, offre des recompenses continues.
Passer à l’action|
Je suis enthousiaste a propos de Ruby Slots Casino, ca invite a plonger dans le fun. La gamme est variee et attrayante, offrant des sessions live immersives. Il booste votre aventure des le depart. Le suivi est impeccable. Les transactions sont toujours securisees, par ailleurs des bonus diversifies seraient un atout. En somme, Ruby Slots Casino est un incontournable pour les joueurs. A signaler le design est tendance et accrocheur, ce qui rend chaque partie plus fun. Egalement genial les tournois reguliers pour s’amuser, qui booste la participation.
Aller sur le site|
makelifebetter – Easy navigation, site layout makes shopping smooth and enjoyable overall.
changetheworld – It would be helpful to know more about the site’s mission and the team behind it.
simplybestchoice – I’d recommend checking the site’s registration age, ownership, and whether it has credible third-party endorsements.
GlobalMarketplaceHub – I enjoy checking daily, lots of interesting new products available.
FindYourPerfectDeal – I keep returning here, always find interesting products quickly and easily.
changeyourworld – Great selection of items, site makes finding deals really easy.
thinkcreategrow – It would be helpful to know more about the site’s mission and the team behind it.
TheBestPlaceToShop – Fast shipping, products arrived on time and in perfect condition today.
startsomethinggreat – Prices are reasonable, found exactly what I wanted without any hassle.
ShopAndSmileAlways – Always something new to discover, makes online shopping fun and exciting.
TrendyFindsHub – Love the variety of products, always find what I need quickly.
learnshareconnect – User-friendly interface that makes navigating and accessing content seamless.
kraken market Kraken ссылка – это ключ к заветной двери, ведущей в глубины цифрового пространства, где правят свои законы. Убедитесь в подлинности источника, прежде чем доверить ему свои данные, ведь мошенники не дремлют. Остерегайтесь фишинговых сайтов и поддельных зеркал, чтобы ваше путешествие не обернулось разочарованием. Будьте бдительны и осмотрительны, и тогда сможете избежать неприятностей и насладиться всеми преимуществами платформы.
findwhatyoulove – Great deals here, always find exactly what I need quickly.
everytrendinone – Customer support was helpful, answered all my questions promptly and politely.
everydayvaluecorner – Fast shipping, products arrived on time and in perfect condition today.
newseasoncollection – Prices are reasonable, found exactly what I wanted without any hassle.
yourjourneybegins – Customer support was helpful, answered all my questions promptly and politely.
discoverendlessideas – I couldn’t find much information about the site’s content or purpose.
ShopTheLatestTrend – Fast shipping, items arrived in perfect condition as expected online.
Hello there! This post couldn’t be written any better! Reading this post reminds me of my good old room mate! He always kept chatting about this. I will forward this article to him. Fairly certain he will have a good read. Many thanks for sharing!
https://parkinsongs.es/melbet-skachat-na-android-besplatno-s-oficialnogo-2025/
smarttradingmentor – The tone here is motivating, feels like genuine mentorship through content.
trendandstyle – The photos are accurate, items look exactly like what’s shown online.
smartbuytoday – I like how clearly items are displayed, makes comparing products so easy.
shoplocaltrend – The site runs smoothly and feels warm, just like a neighborhood shop.
classytrendstore – Great mix of modern and timeless pieces, perfect for daily wear.
freshstylemarket – Love the modern vibe here, everything feels trendy and super well designed.
brightmarketplace – I like how everything is organized, makes finding items really simple.
bestdealcorner – Love the product range here, there’s truly something for every category.
bestdealcorner – Prices are truly unbeatable, found items here much cheaper than elsewhere.
visionpartnersclub – Helpful insights for entrepreneurs, easy to read and apply right away.
discovergreatvalue – The product range here surprised me, covers everything from gadgets to clothes.
classyhomegoods – Great collection of elegant home pieces, ideal for any interior style.
freshfashionfinds – Great photos, easy navigation, and really fresh collections every week.
uniquefashionboutique – I like how everything feels curated, not just random fashion items.
futuregrowthteam – The articles are simple yet powerful, encouraging growth with every read.
sportwetten apps vergleich
Stop by my homepage – Kombiwette Rechner
BuildStrongRelationship – Ideas here have genuinely improved how I connect with others.
protraderacademy – I learned more here in a week than months elsewhere honestly amazing.
yourtradingmentor – Great advice, easy to follow even for people new to trading.
BusinessConnectWorld – Tools are practical and effective, helped me reach new markets.
code promo 1xbet cote d’ivoire 2026 The allure of 1xBet, with its wide array of betting options, is undeniable. However, navigating the world of promo codes can be a labyrinthine task. Whether you seek a “code promo 1xBet” for sports betting, casino games, or simply a “bonus inscription 1xBet,” understanding the landscape is key.
https://directory-boom.com/listings13443146/1xbet-promo-code-for-registration
connectforprogress – I enjoy reading here during breaks, the ideas keep me energized.
purebeautyoutlet – The pictures look so real, makes choosing products much easier honestly.
strongpartnershipnetwork – Truly inspiring platform for anyone wanting to grow through collaboration.
nextleveltrading – Been reading posts here weekly, always find something useful for traders.
tradingmasterclass – Lessons feel real-world focused, easy to apply in actual trades immediately.
MarketTrendAlerts – Highly recommend this platform for anyone tracking market movements closely.
modernvaluecorner – The mix of products here feels trendy yet super affordable overall.
trendinggiftcorner – I like how neatly everything is organized, makes shopping feel smooth.
88fc là nền tảng cá cược thể thao và casino trực tuyến hàng đầu tại châu Á, mang đến trải nghiệm giải trí an toàn và minh bạch cho người chơi.
С каждым годом конкуренция в интернете растёт, и SEO становится ключевым инструментом для успеха. Продвижение помогает занять лидирующие позиции и привлекать новых клиентов без дополнительных затрат на рекламу. Грамотная стратегия SEO укрепляет доверие к бренду и повышает конверсию. Это надёжный путь к росту и развитию. Подробнее о преимуществах оптимизации читайте – http://wiki.fpvfinland.fi/Как_Повысить_Позиции_Сайта_В_Яндексе
Hi there, its good piece of writing about media print, we all be familiar with media is a great source of facts.
https://levacancier.ca/2025/09/23/melbet-ru-promokod-obzor-bonusov-melbet/
Je suis captive par Olympe Casino, ca offre un plaisir melodieux. Il y a une profusion de jeux captivants, incluant des paris sportifs epiques. Avec des depots rapides. Les agents repondent comme des muses, toujours pret a guider. Les paiements sont securises et fluides, parfois plus de promos regulieres ajouteraient de la gloire. Au final, Olympe Casino est une plateforme qui regne sur l’Olympe pour les joueurs en quete d’epopee ! En bonus l’interface est fluide comme un nectar, ce qui rend chaque session plus celeste. Particulierement captivant les tournois reguliers pour la competition, assure des transactions fiables.
https://olympefr.com/|
Are you searching for good working activator for MS Office? On this site you can download the latest version of it: https://artmundo.org/
sportwetten lizenz deutschland
Here is my site; Wette Vorhersage Wetten
вирт секс Вирт модели – это цифровые музы, создающие эстетику и красоту в виртуальном мире. Их образы вдохновляют, восхищают и задают новые стандарты визуального совершенства. Они формируют тренды и влияют на восприятие красоты в онлайн-мире, делая его более ярким и привлекательным.
Доставка пиццы в Туле https://pizzacuba.ru горячо и быстро. Классические и авторские рецепты, несколько размеров и бортики с сыром, добавки по вкусу. Онлайн-меню, акции «2 по цене 1», промокоды. Оплата картой/онлайн, бесконтактная доставка, трекинг заказа.
Ich bin suchtig nach Cat Spins Casino, es ladt zu unvergesslichen Momenten ein. Das Portfolio ist vielfaltig und attraktiv, mit klassischen Tischspielen. Er macht den Einstieg unvergesslich. Der Support ist professionell und schnell. Der Prozess ist transparent und schnell, in seltenen Fallen ein paar zusatzliche Freispiele waren klasse. Zum Schluss, Cat Spins Casino ist ein Muss fur Spieler. Zudem ist das Design stilvoll und einladend, eine immersive Erfahrung ermoglicht. Ein bemerkenswertes Feature die vielfaltigen Wettmoglichkeiten, reibungslose Transaktionen sichern.
Seite erkunden|
Ich liebe das Flair von Cat Spins Casino, es bietet ein mitrei?endes Spielerlebnis. Es gibt zahlreiche spannende Spiele, mit stilvollen Tischspielen. Der Bonus ist wirklich stark. Erreichbar 24/7 per Chat oder E-Mail. Die Zahlungen sind sicher und sofortig, dennoch mehr Aktionen wurden das Erlebnis steigern. Letztlich, Cat Spins Casino sorgt fur ununterbrochenen Spa?. Hinzu kommt die Benutzeroberflache ist flussig und intuitiv, eine Prise Stil hinzufugt. Ein gro?er Pluspunkt die regelma?igen Wettbewerbe fur Spannung, exklusive Boni bieten.
Mehr sehen|
Energy Storage Systems https://e7repower.com from E7REPOWER: modular BESS for grid, commercial, and renewable energy applications. LFP batteries, bidirectional inverters, EMS, BMS, fire suppression. 10/20/40 ft containers, scalable to hundreds of MWh. Peak-saving, balancing, and backup. Engineering and service.
мотосезон2025 ГАИ: Классика, проверенная временем, безопасность превыше всего!
Je suis captive par Sugar Casino, c’est une plateforme qui deborde de dynamisme. Il y a une abondance de jeux excitants, comprenant des jeux optimises pour Bitcoin. Il rend le debut de l’aventure palpitant. Le service d’assistance est au point. Les gains arrivent en un eclair, neanmoins des offres plus importantes seraient super. Dans l’ensemble, Sugar Casino est un must pour les passionnes. Ajoutons que le design est tendance et accrocheur, ce qui rend chaque partie plus fun. Un point cle les paiements securises en crypto, garantit des paiements securises.
Voir la page|
sportwetten geld zurück österreich
my web blog beste wettbüro
Je suis fascine par Sugar Casino, ca transporte dans un univers de plaisirs. La bibliotheque de jeux est captivante, comprenant des jeux crypto-friendly. Il donne un elan excitant. Le support est rapide et professionnel. Le processus est fluide et intuitif, par contre des bonus plus frequents seraient un hit. En resume, Sugar Casino offre une experience hors du commun. Ajoutons que le site est rapide et engageant, permet une plongee totale dans le jeu. Particulierement attrayant les options de paris sportifs diversifiees, assure des transactions fluides.
AccГ©der Г la page|
forexstrategyguide – The tips here feel real and experience-based, not just generic trading advice.
Je suis enthousiaste a propos de Ruby Slots Casino, c’est une plateforme qui deborde de dynamisme. Le catalogue est un tresor de divertissements, offrant des tables live interactives. 100% jusqu’a 500 € plus des tours gratuits. Les agents repondent avec efficacite. Les retraits sont ultra-rapides, de temps en temps des recompenses supplementaires dynamiseraient le tout. En fin de compte, Ruby Slots Casino merite une visite dynamique. En complement le design est moderne et attrayant, apporte une touche d’excitation. Egalement excellent les evenements communautaires dynamiques, offre des recompenses continues.
Visiter pour plus|
brahmanshome – Great website, navigation feels smooth and everything is easy today.
Je suis bluffe par Ruby Slots Casino, il cree une experience captivante. Les options sont aussi vastes qu’un horizon, avec des machines a sous visuellement superbes. 100% jusqu’a 500 € plus des tours gratuits. Disponible a toute heure via chat ou email. Les transactions sont d’une fiabilite absolue, neanmoins des bonus plus frequents seraient un hit. En bref, Ruby Slots Casino offre une experience hors du commun. En plus le design est tendance et accrocheur, ce qui rend chaque session plus excitante. Un avantage les evenements communautaires dynamiques, propose des privileges personnalises.
Visiter la plateforme|
everydaytrendshop – Customer support was super responsive and helpful during my checkout.
Moldova – rent-auto.md/ro/ – Inchiriere auto Chisinau – arenda masini fara stres, rezervare rapida si cele mai bune preturi.
yourtradingmentor – Everything is clearly structured, helps build confidence with every new topic.
teamworkinnovation – Helpful advice for modern workplaces looking to boost productivity together.
globalchoicehub – Love the concept of connecting shoppers worldwide with great product choices.
simplelivinghub – I like how every item feels purposeful, not just another random thing.
бк мелбет официальный сайт [url=https://melbetofficialsite.ru]бк мелбет официальный сайт[/url] .
shopandshine – Love the name, and the site really matches that bright, polished energy.
teamworksuccesspath – Love how they focus on shared growth and mutual understanding in business.
successnetworkgroup – The content feels empowering, full of positivity and practical growth tips.
uniquedecorstore – The aesthetics and design of the products really stand out online and offline.
globalmarketinsight – I like the clean layout, makes reading reports and updates super smooth.
successfultradersclub – Great platform for traders looking to improve consistently with structured guidance.
markettrendalerts – The layout is simple and clean, makes monitoring trends much faster.
На сайте https://fotoredaktor.top узнал, на каком языке слово чиназес.
Академия Алины Аблязовой https://ablyazovaschool.ru обучение реконструкции волос для мастеров и новичков. Авторские методики, разбор трихологических основ, отработка на моделях, кейсы клиентов. Онлайн и офлайн, сертификат, поддержка кураторов, материалы и чек-листы.
learnandtrade – I’ve learned a lot about risk management just from a few sessions here.
Hi there! Do you use Twitter? I’d like to follow you if that would be okay. I’m definitely enjoying your blog and look forward to new posts.
номера вирт
jonathanfinngamino – Really helpful tips here, saved me hours of unnecessary work.
новости мобильных игр Во что поиграть: Убей время с пользой!
wetten ergebnisse tipps
My webpage; wettbüro duisburg
Pretty section of content. I just stumbled upon your site and in accession capital to assert that
I get in fact loved account your weblog posts. Anyway I will be subscribing in your augment
or even I success you access consistently rapidly.
halbzeit endstand wette erklärung
Also visit my site … erfahrungen wett tipps ai (Fausto)
paws21airbrushstudio – The attention to detail in their artwork is remarkable.
Продвижение сайта помогает привлекать новых клиентов и повышать доверие аудитории. Оптимизация включает работу с контентом, структурой и техническими аспектами ресурса. Регулярная аналитика и обновление информации позволяют поддерживать позиции и обеспечивать стабильный рост. В результате сайт становится удобным и полезным для пользователей. Такой подход обеспечивает долгосрочное развитие компании. Читайте подробнее http://leadwith.org/bbs/board.php?bo_table=free&wr_id=404279
motocitee – Site layout is clean, making it very easy to follow instructions.
It’s clear you’re passionate about the issues.
kivurc – Love browsing this site, content is always practical and clear.
kraken ссылка Kraken сайт – это таинственный портал в мир эксклюзивных возможностей, где анонимность и безопасность возведены в абсолют. Здесь границы стираются, а мечты обретают реальность, но помните: с большой свободой приходит и большая ответственность. Каждый шаг требует осознанности и понимания, ведь за порогом скрываются не только сокровища, но и подводные камни. Подходите к изучению платформы с мудростью и осторожностью, взвешивая каждый свой выбор.
raspinakala – Content is fresh, practical, and engaging for anyone working at home.
Оптимизация сайта под поисковые системы помогает увеличить посещаемость ресурса и повысить конверсию. SEO делает сайт удобным для пользователей и понятным для поисковых систем. Комплексная работа с контентом, метатегами и техническими настройками обеспечивает долгосрочный результат. Такой подход снижает зависимость от рекламы и укрепляет позиции компании. Подробнее о продвижении читайте, https://oerdigamers.info/index.php/Сео_Продвижение_Сайтов_Для_Предпринимателей_И_Фирм
adawebcreative – Clean design, easy navigation, and informative content. Highly recommend.
ibdesignstreet – Love browsing this site, content is always practical and clear.
pgmbconsultancy – Great website, navigation feels smooth and everything is easy today.
abbysauce – Helpful ideas posted here, I’ll definitely share with all friends.
wooziewear – Site layout is clean, making it very easy to follow instructions.
expandyourhorizon – A great resource for staying updated on various topics.
SEO помогает сделать сайт не просто красивым, но и эффективным. Оптимизация повышает доверие поисковых систем и улучшает конверсию. Благодаря комплексной работе результат сохраняется надолго. Этот инструмент приносит устойчивый трафик. Узнайте больше о продвижении https://online-learning-initiative.org/wiki/index.php/User:Clement24X
inkorswimtattoocruise – Found exactly what I needed, instructions were clear and concise.
lotsofonlinepeople – Navigation is smooth, everything appears exactly where I expected it.
oldschoolopen – Enjoyed reading these posts, ideas are simple yet very useful.
letter4reform – I appreciate the clarity and depth of their content.
chopchopgrubshop – The ambiance complements the flavorful dishes perfectly, creating a memorable experience.
zz-meta – Excellent explanations, made complex topics feel much easier instantly.
chopchopgrubshop – A hidden gem with unique flavors and great service.
ieeb – A great resource for staying updated on current trends.
ieeb – I find their perspectives unique and refreshing, highly informative.
lastminute-corporate – The site offers seamless booking experiences for corporate travelers.
broodbase – I find their perspectives unique and refreshing, highly informative.
kazhanmaster – The content is diverse and engaging, always something new to explore.
conorjmurphy – Great website, navigation feels smooth and everything is easy today.
sjydtech – Their layout is sharp, navigation feels intuitive and modern.
Оптимизация сайта под поисковые системы — это важный этап в развитии любого онлайн-бизнеса. SEO помогает улучшить позиции ресурса и повысить доверие пользователей. Благодаря правильной настройке и анализу ключевых запросов сайт становится более видимым для целевой аудитории. Это не быстрый, но надёжный инструмент для роста. Подробнее о стратегиях SEO можно узнать https://45.76.249.136/index.php?title=Создание_Сайтов_И_Их_Сео_Продвижение
блог seo агентства [url=http://www.statyi-o-marketinge7.ru]http://www.statyi-o-marketinge7.ru[/url] .
bester neukundenbonus wettanbieter
My site … Paysafecard sportwetten
Автоматические выключатели
Ich bin begeistert von der Welt bei Cat Spins Casino, es verspricht pure Spannung. Die Spielesammlung ist uberwaltigend, mit eleganten Tischspielen. Mit sofortigen Einzahlungen. Der Kundensupport ist erstklassig. Transaktionen laufen reibungslos, trotzdem gro?zugigere Angebote waren klasse. Alles in allem, Cat Spins Casino ist eine Plattform, die uberzeugt. Zusatzlich die Navigation ist klar und flussig, das Spielvergnugen steigert. Ein besonders cooles Feature die dynamischen Community-Veranstaltungen, die die Community enger zusammenschwei?en.
Jetzt beginnen|
Ich freue mich auf Cat Spins Casino, es ist ein Ort, der begeistert. Das Portfolio ist vielfaltig und attraktiv, mit klassischen Tischspielen. Er steigert das Spielvergnugen sofort. Der Service ist immer zuverlassig. Zahlungen sind sicher und schnell, von Zeit zu Zeit mehr Bonusvielfalt ware ein Vorteil. Kurz und bundig, Cat Spins Casino ist ein Muss fur Spielbegeisterte. Nebenbei die Navigation ist unkompliziert, einen Hauch von Eleganz hinzufugt. Ein bemerkenswertes Feature die zahlreichen Sportwetten-Moglichkeiten, das die Motivation steigert.
Jetzt zugreifen|
Ich bin fasziniert von Cat Spins Casino, es bietet ein mitrei?endes Spielerlebnis. Die Auswahl ist einfach unschlagbar, mit interaktiven Live-Spielen. Der Willkommensbonus ist ein Highlight. Der Support ist schnell und freundlich. Transaktionen laufen reibungslos, trotzdem regelma?igere Promos wurden das Spiel aufwerten. Alles in allem, Cat Spins Casino ist ein Muss fur Spielbegeisterte. Ubrigens die Navigation ist klar und flussig, zum Verweilen einladt. Ein klasse Bonus die lebendigen Community-Events, schnelle Zahlungen garantieren.
Jetzt beitreten|
Ich bin ein gro?er Fan von Cat Spins Casino, es ladt zu unvergesslichen Momenten ein. Das Angebot ist ein Traum fur Spieler, mit Spielautomaten in beeindruckenden Designs. Mit schnellen Einzahlungen. Der Support ist professionell und schnell. Der Prozess ist unkompliziert, jedoch mehr Bonusangebote waren spitze. Im Gro?en und Ganzen, Cat Spins Casino ist ein Top-Ziel fur Spieler. Zusatzlich die Seite ist zugig und ansprechend, das Spielvergnugen steigert. Besonders erwahnenswert die vielfaltigen Wettmoglichkeiten, die Teilnahme fordern.
Jetzt zugreifen|
J’adore le dynamisme de Sugar Casino, il procure une sensation de frisson. La variete des jeux est epoustouflante, avec des machines a sous visuellement superbes. Il amplifie le plaisir des l’entree. Le suivi est toujours au top. Les gains arrivent en un eclair, parfois plus de promotions frequentes boosteraient l’experience. En bref, Sugar Casino est un endroit qui electrise. Ajoutons que le site est rapide et engageant, ce qui rend chaque session plus excitante. Particulierement cool les nombreuses options de paris sportifs, garantit des paiements rapides.
http://www.sugarcasinobonus777fr.com|
Je suis fascine par Ruby Slots Casino, ca offre un plaisir vibrant. Les options sont aussi vastes qu’un horizon, comprenant des jeux crypto-friendly. Avec des depots fluides. Disponible 24/7 pour toute question. Le processus est simple et transparent, a l’occasion quelques free spins en plus seraient bienvenus. Pour finir, Ruby Slots Casino assure un fun constant. Notons aussi l’interface est fluide comme une soiree, ce qui rend chaque session plus palpitante. A noter les evenements communautaires dynamiques, renforce la communaute.
Rejoindre maintenant|
J’adore la vibe de Sugar Casino, ca transporte dans un monde d’excitation. La bibliotheque est pleine de surprises, offrant des sessions live immersives. Avec des transactions rapides. Les agents repondent avec rapidite. Le processus est fluide et intuitif, en revanche quelques tours gratuits en plus seraient geniaux. Au final, Sugar Casino est un choix parfait pour les joueurs. Notons aussi l’interface est fluide comme une soiree, incite a prolonger le plaisir. Un bonus le programme VIP avec des recompenses exclusives, renforce le lien communautaire.
Rejoindre maintenant|
J’ai une affection particuliere pour Ruby Slots Casino, c’est une plateforme qui deborde de dynamisme. Les titres proposes sont d’une richesse folle, comprenant des jeux crypto-friendly. Le bonus initial est super. Disponible 24/7 par chat ou email. Les retraits sont fluides et rapides, a l’occasion quelques tours gratuits supplementaires seraient cool. En conclusion, Ruby Slots Casino vaut une visite excitante. Notons egalement la navigation est fluide et facile, ce qui rend chaque partie plus fun. Egalement super les tournois frequents pour l’adrenaline, offre des recompenses continues.
Voir la page d’accueil|
замена стекла Ремонт стекол – это экономичный способ восстановить первоначальный вид и прочность автомобильных стекол. Небольшие сколы, трещины и царапины могут быть устранены опытными мастерами с использованием современных технологий. Вовремя выполненный ремонт предотвращает дальнейшее разрушение стекла и обеспечивает безопасность на дороге.
J’adore la vibe de Sugar Casino, il offre une experience dynamique. Il y a une abondance de jeux excitants, offrant des experiences de casino en direct. Avec des depots rapides et faciles. Le suivi est d’une fiabilite exemplaire. Les transactions sont d’une fiabilite absolue, cependant des offres plus importantes seraient super. En bref, Sugar Casino offre une experience hors du commun. Notons aussi l’interface est lisse et agreable, ce qui rend chaque session plus excitante. Un element fort les tournois frequents pour l’adrenaline, renforce le lien communautaire.
Voir plus|
styleforliving – Delivery info appears transparent which gives me more confidence.
buildabetterworld – Checkout process was seamless and payment options are convenient.
There’s definately a lot to know about this topic.
I like all the points you’ve made.
everydaystylecorner – Overall positive first impression; excited to see how quality and delivery turn out.
kraken market Kraken сайт – это зеркало цифровой эпохи, отражающее как светлые, так и темные её стороны. Это территория, где стираются границы между реальностью и иллюзией, где власть обретает анонимность, а свобода граничит с опасностью. Здесь царят свои законы и правила, требующие от каждого участника не только осведомленности, но и ответственности за свои действия. Погружение в этот мир требует хладнокровия, осторожности и умения видеть за заманчивыми предложениями потенциальные риски.
discovernewthings – Overall good first impression; hoping delivery and quality match expectations.
makelifesimpler – Consistently useful content that didn’t feel overwhelming at all.
https://viploggias.ru/ Ремонт стекол – это комплекс процедур, направленных на восстановление целостности и прозрачности автомобильных стекол. Он включает в себя ремонт сколов, трещин, а также полировку для устранения царапин и потертостей. Своевременный ремонт позволяет избежать дорогостоящей замены стекла и обеспечивает безопасность вождения.
yourcreativejourney – I appreciated the visible shipping/returns info—it gives trust.
theworldawaits – Quality product visuals make me confident about placing an order.
Пенсионерам https://pensioneram.in.ua все о начилении пенсии, пособиях и выплатах в Украине. Полезные ссылки, законы и права пенсионеров и инвалидов.
discovergreatdeals – The offers caught my eye quickly—worth checking regularly.
learnandgrowtoday – I’m looking forward to exploring more of the topics available.
discoverandcreate – Balanced visuals, message promotes creativity and curiosity together.
https://ritual-uslugi-spb-reg.ru/
My brother recommended I would possibly like this blog. He was once totally right.
This put up truly made my day. You can not believe just how much time I
had spent for this info! Thank you!
sportwetten ergebnisse vorhersage
my web site :: Sportwette
bestvaluecollection – Delivery and quality will be the real test but initial feel is promising.
обзор changan uni https://changan-v-spb.ru
ЛідерUA – інформативний портал https://liderua.com новин та корисних порад: актуальні події України, аналітика, життєві лайфхаки та експертні рекомендації. Все — щоб бути в курсі й отримувати практичні рішення для щоденного життя та розвитку.
seo бесплатно [url=http://kursy-seo-11.ru]seo бесплатно[/url] .
buildabetterworld – Uplifting concept, site feels full of purpose and positivity.
Главное у нас: https://journal-ua.com/kulinariia/yak-osvojiti-vashu-dukhovu-shafu-rezhimi-funktsiji-ta-sekreti-doskonalikh-strav.html
changeyourdirection – If delivery and product durability match this strong start, this could be a solid find.
growwithconfidence – I like how supportive and approachable the tone of the site is.
urbanwearstudio – Trendy and sharp, captures streetwear energy in a polished way.
getinspireddaily – Found some items I liked—delivery and support will tell the full story.
globalstylemarket – Customer support contact appears available, which builds confidence in ordering.
Курсы маникюра https://econogti-school.ru и педикюра с нуля: теория + практика на моделях, стерилизация, архитектура ногтя, комбинированный/аппаратный маникюр, выравнивание, покрытие гель-лаком, классический и аппаратный педикюр. Малые группы, материалы включены, сертификат и помощь с трудоустройством.
Авторский MINI TATTOO https://kurs-mini-tattoo.ru дизайн маленьких тату, баланс и масштаб, безопасная стерилизация, грамотная анестезия, техника fine line и dotwork. Практика, разбор типовых косяков, правила ухода, фото/видео-съёмка работ. Материалы включены, сертификат и поддержка сообщества.
uniquelifestylehub – Checkout appeared straightforward and payment options were clearly displayed.
connectlearncreate – So far a good first impression—looking forward to seeing how it evolves.
exploreandcreate – Customer support information seems accessible which adds confidence.
fashionloverszone – Customer support/contact information looks accessible, which builds trust.
Зефірка https://zefirka.net.ua сонники, значение имен и прздники. Развлекательный блог с приметами и советами
7к казино игровые автоматы 7к известны высокими выплатами и интересными сюжетами, что привлекает множество игроков.
connectwithideas – Customer support info is accessible which boosts trust in the experience.
Максимум качества, минимум травм. Профессиональные наборы для татуировки: машинки, блоки питания, премиум-краски. Всё для стерильной и крутой работы. Обновите свой арсенал сегодня https://tattoo-sale.ru/
makeimpacttoday – I’ll bookmark this shop for future visits when I’m ready to shop.
dreambuyoutlet – Overall first impression is strong; excited to see how this store performs.
sportwetten forum strategie
My website – perfekte wettstrategie; https://servaco.com.br,
discoveryourpotential – Navigation appears smooth and product or service categories seem clear.
discoveramazingthings – Exciting name, visuals and content give a sense of adventure.
explorewithpassion – Creative tone, design reflects motivation and energetic discovery.
kraken сайт Kraken market – это шумный восточный базар, перенесенный в виртуальную реальность. Здесь можно найти всё, что угодно, от редких артефактов до запрещённых веществ. Однако, не стоит терять голову от разнообразия предложений и низких цен. Внимательно изучайте отзывы о продавцах, проверяйте информацию о товарах и не стесняйтесь задавать вопросы. Только так вы сможете совершить выгодную и безопасную сделку, избежав разочарований и проблем с законом. Будьте мудрыми и предусмотрительными, и Kraken market откроет перед вами свои сокровища.
modernhomefinds – Customer support/contact info seems accessible, which helps build trust.
findyourdreamplace – The checkout process appears straightforward and payment options are visible.
discoveramazingoffers.shop – Love checking daily, offers always keep me coming back quickly.
smartshoppinghub – Clean and practical, ideal for modern online shoppers and deals.
Онлайн-блог https://intellector-school.ru о нейросетях: от базовой линейной алгебры до Transformer и LLM. Пошаговые проекты, код на Git-стиле, эксперименты, метрики, тюнинг гиперпараметров, ускорение на GPU. Обзоры курсов, книг и инструментов, подборка задач для практики и подготовки к интервью.
Освойте режиссуру https://rasputinacademy.ru событий и маркетинг: концепция, сценарий, сцена и свет, звук, видео, интерактив. Бюджет и смета, закупки, подрядчики, тайминг, риск-менеджмент. Коммьюнити, PR, лидогенерация, спонсорские пакеты, метрики ROI/ROMI. Практические задания и шаблоны документов.
urbanwearstudio – The product range looks appealing—some standout pieces in the roster.
imaginelearnexplore – Customer support or contact info appears accessible, which adds trust.
discoverandcreate – Overall a positive outlook; excited to see how the site grows.
purefashionmarket – Found some interesting fashion pieces that seem thoughtfully selected.
yourmomenttoshine – The overall vibe is clean and modern; a solid first impression.
discovernewthings – Bright visuals and curious tone make this site engaging.
Play The Password Game online in Canada! Test your creativity and problem-solving skills as you craft secure passwords with fun, unique challenges. Perfect for puzzle enthusiasts: classic Password board game online
explorewithpassion.shop – Always something new to discover here, feels fresh and exciting.
kraken ссылка Kraken сайт – это зеркало цифровой эпохи, отражающее как светлые, так и темные её стороны. Это территория, где стираются границы между реальностью и иллюзией, где власть обретает анонимность, а свобода граничит с опасностью. Здесь царят свои законы и правила, требующие от каждого участника не только осведомленности, но и ответственности за свои действия. Погружение в этот мир требует хладнокровия, осторожности и умения видеть за заманчивыми предложениями потенциальные риски.
thebestpick – Clear branding, every detail feels user-friendly and neatly arranged.
changeyourperspective – The checkout process appears straightforward and payment methods are clearly displayed.
changeyourperspective.click – Love how this site challenges my mindset in refreshing ways today.
createyourfuture.click – Inspiring site overall, helps me stay motivated toward long-term goals.
Cocaine comes from coca leaves establish in South America.
While periodically cast-off in traditional pharmaceutical,
it’s in this day a banned core due to its dangers.
It’s hugely addictive, unrivalled to well-being risks like pump attacks,
mentally ill disorders, and mean addiction.
modernhomefinds.click – Love the stylish home ideas here, everything feels fresh and cozy.
shopthetrendtoday – Stylish layout, ideal for trendy products and fast-fashion finds.
Thanks for another fantastic article. Where else may
anyone get that type of information in such a perfect way of writing?
I have a presentation next week, and I am at the search for such info.
findjoyeveryday.shop – Simple layout, fun products, everything feels bright and positive today.
findyourmoments.click – Great message here, every visit feels grounding and truly refreshing.
yourdailyshoppinghub.shop – Really convenient website, makes everyday shopping fast and stress-free always.
classyfindsonline.shop – Love the elegant feel here, every product looks thoughtfully selected.
trendinghomeideas.shop – Love the minimalist style, makes redecorating feel easy and affordable.
buildsomethingnew.shop – Love the concept here, inspires creativity and hands-on learning daily.
simplevalueplace.shop – Excellent value overall, definitely bookmarking this for future purchases.
smartfashionboutique.shop – Love this shop’s personality, everything feels chic and thoughtfully made.
startanewjourney.shop – Great concept overall, inspires motivation and personal transformation beautifully.
trendingstyleworld.shop – Love this boutique vibe, everything screams fashion-forward and effortlessly trendy.
modernlivingtrend.shop – Great collection of stylish pieces, perfect for updating any home.
changeyourperspective.click – Love how this site challenges my mindset in refreshing ways today.
findyourpathnow.shop – Love the motivation here, makes me feel more grounded every day.
staycuriousdiscover.shop – Love how thought-provoking this site feels, very refreshing and creative.
yourmomentstarts.click – The design feels calm and motivating, perfect start to any day.
modernlivingstore.click – Amazing store layout, easy to navigate and full of inspiration.
createyourfuture.click – Inspiring site overall, helps me stay motivated toward long-term goals.
modernhomefinds.click – Love the stylish home ideas here, everything feels fresh and cozy.
Pretty nice post. I just stumbled upon your blog and wished to say that
I’ve truly enjoyed surfing around your blog posts.
In any case I’ll be subscribing to your feed and I hope you write again soon!
freshfashioncorner.shop – Found the perfect jacket here, quality feels amazing for price.
discoveryourpotential.click – Great platform for personal growth, love the positive and uplifting tone.
Курсы по наращиванию https://schoollegoart.ru ресниц, архитектуре и ламинированию бровей/ресниц с нуля: теория + практика на моделях, стерильность, карта бровей, классика/2D–4D, составы и противопоказания. Материалы включены, мини-группы, сертификат, чек-листы и помощь с портфолио и стартом продаж.
yourmomentstarts.shop – This platform’s energy is great, makes every visit feel refreshing.
PRP-курс для косметологов плазмотерапия обучение в москве доказательная база, отбор пациентов, подготовка образца, техники введения (лицо, шея, кожа головы), сочетание с мезо/микронидлингом. Практика, рекомендации по фото/видео-фиксации, юридические формы, маркетинг услуги. Сертификат и кураторство.
findsomethinggreat.click – Great place to browse, always end up discovering something unexpectedly cool.
classychoiceoutlet.click – Stylish collection overall, love the elegant and affordable fashion pieces.