
Course : CBSE Class 10 Science-Detailed Notes
Empower your CBSE Class 10 Science preparation with our comprehensive online course, featuring detailed and informative notes! This course is meticulously designed to provide you[…]

CBSE Class 10 Science 2023-24 : Important Questions ( SQP-1)
CBSE 10th exams are currently underway, with the Science paper scheduled for 2nd March 2024. With only a few hours remaining before the Science exam,[…]
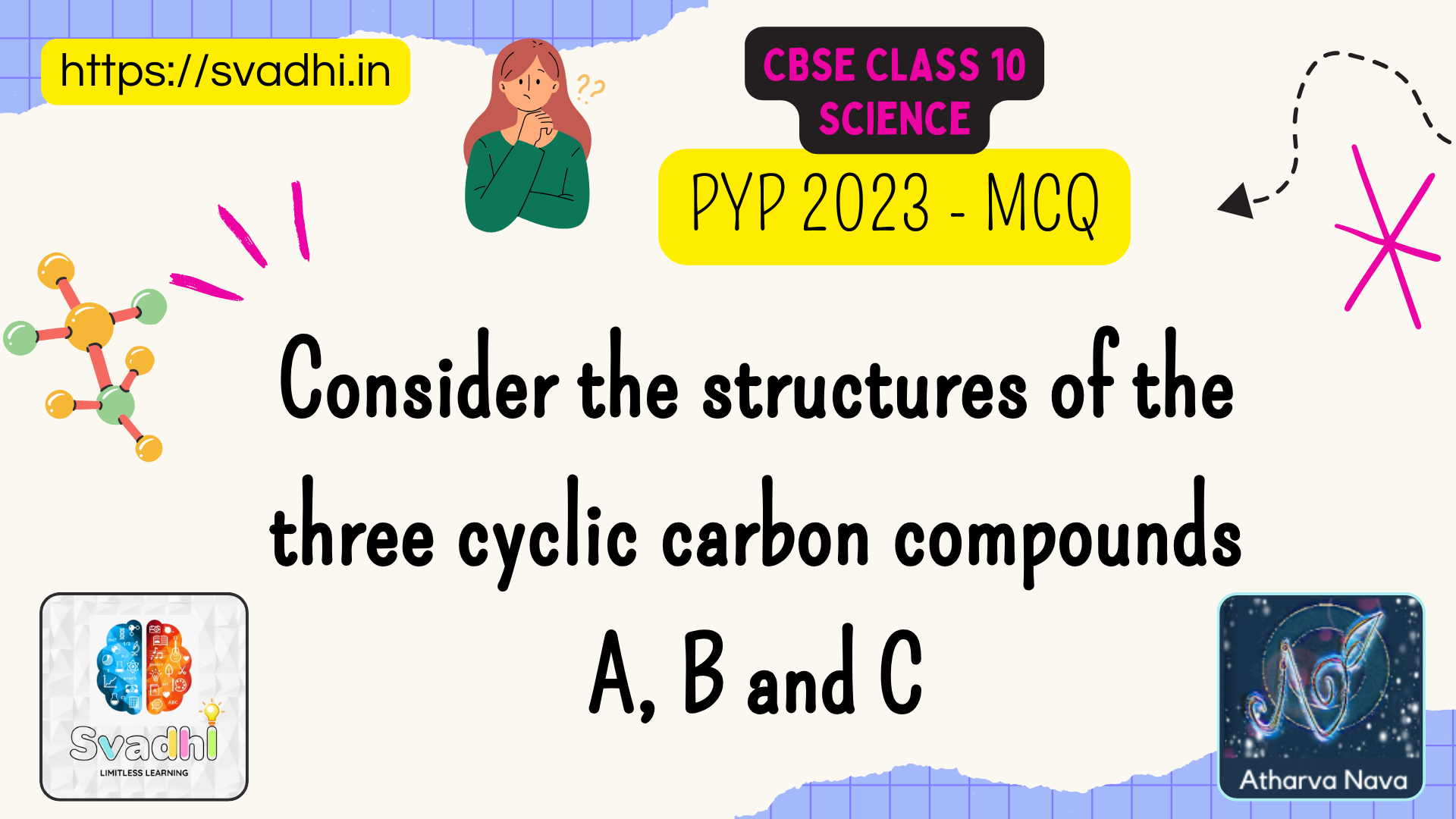
Consider the structures of the three cyclic carbon compounds
https://youtu.be/kKQP5eyhqD0 A, B and C given below and select the correct option from the following: Analysis of the figures: The three structures shown in[…]

The table shows the pH and nature (acidic/basic) of four different solutions
https://youtu.be/BgYBoiykPgs Which one of the options in the table is correct ? Option Solution Colour of pH paper Approximate pH value Nature of solution (a)[…]

A metal ‘X’ is used in thermite process. When X is burnt in air it gives an amphoteric oxide ‘Y’. ‘X’ and ‘Y’ are respectively :
https://www.youtube.com/watch?v=RD6qPT9rf3g Explanation: Here, the metal used in the thermite process is aluminium (Al). When aluminium is burned in air, it produces aluminium oxide (Al₂O₃), which[…]
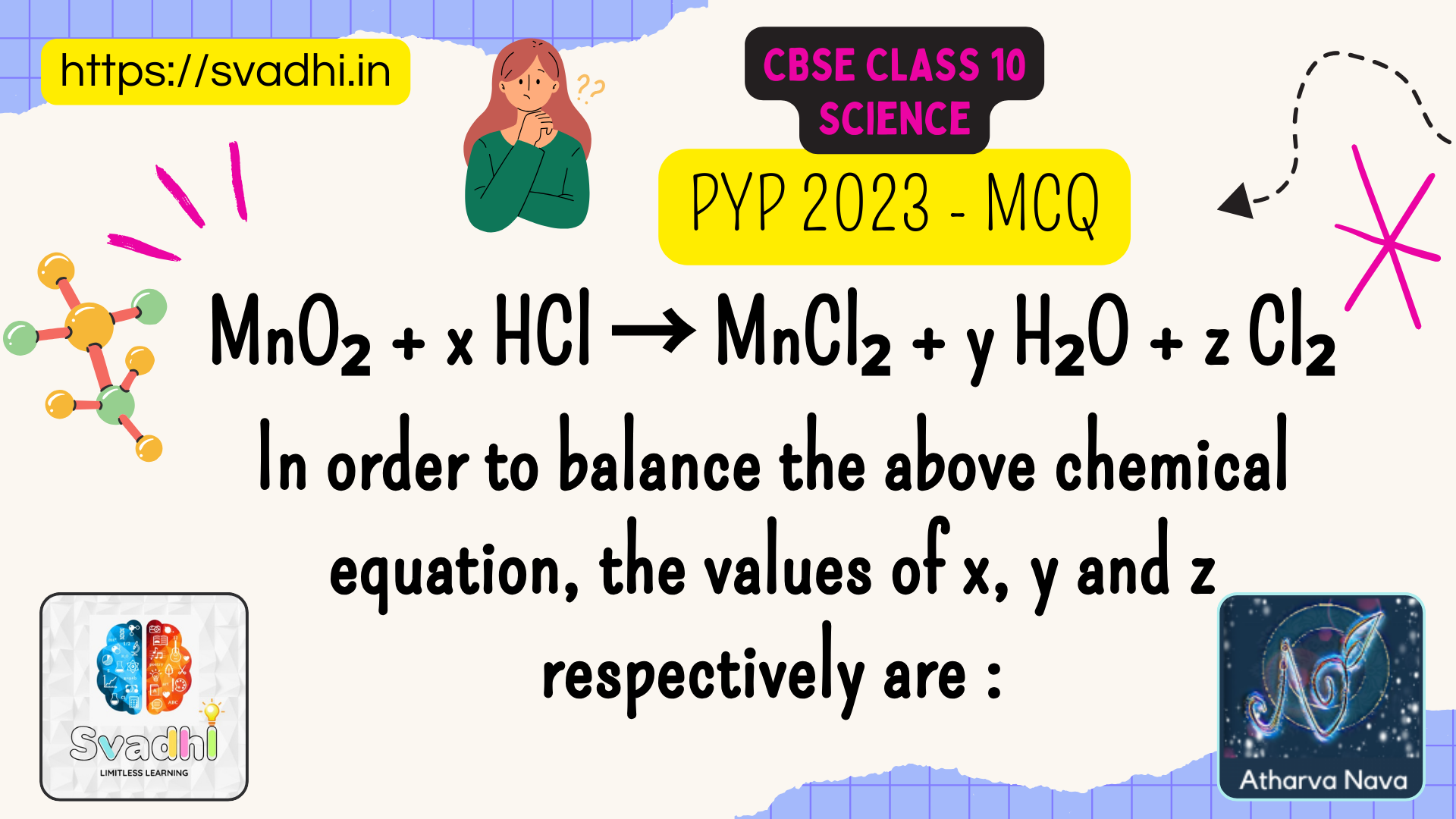
MnO₂ + x HCl → MnCl₂ + y H₂O + z Cl₂
In order to balance the above chemical equation, the values of x, y and z respectively are : a) 6, 2, 2 b) 4, 1,[…]

The emission of brown fumes in the given experimental set-up is due to
https://youtu.be/YwyP-bDU6Tk Here is the Explanation: The correct option is (a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide. When lead nitrate[…]

In the experimental setup given below, it is observed that on passing the gas produced in the reaction in the solution X, the solution X first turns milky and then colourless.
https://youtu.be/V6Hg_xyNO_k The option that justifies the above stated observation is that ‘X’ is aqueous calcium hydroxide and (a) it turns milky due to carbon dioxide[…]

Name the functional group present in propanone
The functional group present in propanone is a ketone. Here’s a breakdown of what that means, Think of it like this: Another example of a[…]

Alkane of 11 carbon in ring structure : Molecular formula
https://www.youtube.com/watch?v=Cps4B-x4L_4 An alkane has 11 carbon atoms arranged within ring structures as shown below. what is the molecular formula of the alkane? The alkane in[…]