MCQ’S
I. Choose the correct answer from the given options.
1. In the long form of periodic table the alkaline earth metals are placed in:
(a) group 17
(b) group 18
(c) group 2
(d) group 1
Ans: (c) group 2
2. Which one is a metalloid?
(a) aluminium
(b) silicon
(c) sulphur
(d) carbon
Ans: (b) silicon
3. On the basis of electron configuration of valence shell, which one is odd
(a) argon
(b) krypton
(c) helium
(d) neon
Ans: (c) helium
4. The property which is not the characteristic of covalent compound is that:
(a) it vaporises easily
(b) it has a low melting point
(c) it is a weak electrolyte
(d) it often exist in liquid state
Ans: (c) it is a weak electrolyte
5. Amongst the elements given below, the element with least electronegativity is:
(a) carbon
(b) fluorine
(c) boron
(d) lithium
Ans: (d) lithium
6. The most stable electron configuration of valence shell of an atom is:
(a) duplet configuration
(b) octet configuration
(c) both (a) and (b)
(d) none of these
Ans: (c) both (a) and (b)
7. Which is a hygroscopic substance?
(a) Conc sulphuric acid
(b) Calcium oxide
(c) Phosphorus penta-oxide
(d) All of these
Ans: (d) All of these
8. The degree of ionisation of an acid is called:
(a) strength of an acid
(b) concentration of an acid
(c) combining capacity of an acid
(d) both (a) and (b)
Ans: (a) strength of an acid
9. A chloride which forms a bluish white precipitate that is soluble in excess of ammonium hydroxide, is:
(a) Calcium chloride
(b) Ferrous chloride
(c) Ferric chloride
(d) Copper Chloride
Ans: (d) Copper Chloride
10. The chloride of a metal which is insoluble in cold water, but soluble in hot water.
(a) silver chloride
(b) copper (II) chloride
(c) lead (II) chloride
(d) iron (II) chloride
Ans: (c) lead (II) chloride
11. Whenever the gases react under similar conditions of temperature and pressure they do so in volumes, which bear a simple ratio of each other, and the products if gaseous. The above law was stated by:
(a) Boyle
(b) Charle’s
(c) Gay Lussac
(d) Avogadro
Ans: (c) Gay Lussac
12. The ion which most readily discharges at cathode is:
(a) Cu2+
(b) H+
(c) Fe2+
(d) Al3+
Ans: (a) Cu2+
13. The metallic electrode which does not take part in electrolytic reaction is:
(a) copper
(b) platinum
(c) nickel
(d) silver
Ans: (b) platinum
14. Elements in molten state, on mixing and the cooling form a homogeneous called alloy are:
(a) metals
(b) non-metals
(c) metalloids
(d) all of these
Ans: (d) all of these
15. The number of valence electrons in metals are:
(a) 1 to 2
(b) 4 to 6
(c) 1 to 3
(d) 4 to 7
Ans: (c) 1 to 3
16. The metals are generally:
(a) oxidising agents
(b) reducing agents
(c) hardening agents
(d) catalytic agents
Ans: (b) reducing agents
17. The gastric juice secreted by the walls of the stomach contains:
(a) sulphuric acid
(b) hydrochloric acid
(c) nitric acid
(d) acetic acid
Ans: (b) hydrochloric acid
18. When the equal volumes of hydrogen gas and chlorine gas are exposed to diffused sunlight, the reaction:
(a) does not take place
(b) takes place at moderate speed
(c) is explosive in nature
(d) none of these
Ans: (b) takes place at moderate speed
19. A well known compound of ammonia which occurs naturally is “sal ammoniac”. The chemical name of this compound is:
(a) ammonium sulphate
(b) ammonium chloride
(c) ammonium nitrate
(d) ammonium carbonate
Ans: (b) ammonium chloride
20. Common name of conc. sulphuric acid is:
(a) aqua fortis
(b) aqua regia
(c) oil of vitriol
(d) green vitriol
Ans: (c) oil of vitriol
2 Marks Questions
1. (i) Name three alkali metals and state their group number.
(ii) Name three alkaline earth metals and state their group number.
(iii) Name three halogens and state their group number.
(iv) Name three noble gases and state their group.
Ans. (i) Three alkali metals are lithium, sodium and potassium. They belong to group IA.
(ii) Three alkaline earth metals are beryllium, magnesium and calcium. They belong to group II A.
(iii) Three halogens are fluorine, chlorine and bromine. They belong to VII A group.
(iv) Three noble gases are helium, neon and argon. They belong to the zero group.
2. (i) What do you understand by the term “transition elements”?
(ii) Select transition elements from the following list:
List: potassium, calcium, manganese, chromium, copper, calcium, iron, platinum.
Ans. (i) The elements which have valence shell and the shell next to it incomplete are called transition elements.
(ii) Manganese, chromium, copper, iron and platinum are transition elements.
3. What is the charge on a chloride ion? How does this charge come about?
Ans. (i) The chloride ion has unit negative charge.
(ii) When the chlorine atom (2, 8, 7) accepts one electron so as to have a stable argon like structure, there is one electron in excess, as compared to the number of protons in the nucleus. This in turn makes chloride ion negatively charged.
4. Why are metals good conductor of electricity?
Ans. All metals have one to three electrons in their valence shell, which are very loosely held by the nucleus. When an electric p.d. is applied, these free electrons start drifting in one particular direction, thereby making the metals good conductor of electricity.
5. On what basis are the strength of (i) acids, (ii) alkalis determined?
Ans. (i) The strength of an acid is determined by the concentration of hydronium ions present in its aqueous solution.
(ii) The strength of an alkali is determined by the concentration of hydoxyl ions present in its solution.
6. Differentiate between a strong acid and weak acid. Give two examples.
Ans. An acid which dissociates almost completely in an aqueous solution, thereby producing high concentration of H– (aq) ions is called a strong acid. For example, hydrochloric acid, nitric acid, sulphuric acid and phosphoric acid.
An acid which dissociates only partially in an aqueous solution thereby producing low concentration of H+(aq) ions is called a weak acid. For example, acetic acid, carbonic acid, sulphurous acid, nitrous acid are weak acids.
7. Given: 2C2H6 +702 → 4CO2 + 6H2O
2000 cc of O2 was burnt with 400 cc of ethane.
Calculate the volume of CO2 formed and unused O2.
Ans:


8. (i) State Gay Lussac’s Law of combining gases.
(ii) Nitrogen and oxygen react as illustrated by an equation below :
N2(g) + O2(g) → 2NO
Calculate the volume of nitrogen gas to produce 5.6 dm³ of nitric oxide gas at S.T.P.
Ans: (i) Gay Lussac’s Law: Whenever the gases react chemically, they do so in volumes which bear a simple whole number ratio to one another and the products, if gaseous, provided the gases are at same temperature and pressure.

9. (a) What do you understand by the term electrolytic dissociation?
(b) The ionisation of sodium chloride in water is 99%. What do you mean by the statement?
Ans. (a) Electrolytic dissociation : The process in which separation of ions of an ionic compound takes place on heating or in an aqueous solution is called electrolytic dissociation.
(b) It means that at any time 99 molecules of sodium chloride are in an ionised state, out of 100 molecules of sodium chloride.
10. Explain, why a solution of an ionic compound is an electrolyte while that of a covalent compound is a non- electrolyte.
Ans. The solution of an ionic compound has free ions which can migrate to the cathode and anode and discharge. Thus, a solution of an ionic compound is a good conductor of electricity, and hence is an electrolyte. However, a solution of a covalent compound, consists of only molecules and does not have any free ions, which can migrate to the cathode or anode. Hence, it is a non-electrolyte.
11. Name the following:
(a) The property possessed by metals by which they can be beaten into sheets.
(b) A compound added to lower the fusion temperature of electrolytic bath in the extraction of aluminium.
Ans. (a) Malleability
(b) Cryolite
12. What is meant by the term metallurgy? Differentiate between a mineral and ore?
Ans. Metallurgy: The different processes involved in the extraction of pure metals from their ores are collectively called metallurgy. Any metallic or non-metallic compounds occurring in nature is called a mineral, whereas a metallic mineral, from which a metal can be profitably extracted, is called an ore.
13. (a) What is the property of concentrated sulphuric acid which allows it to be used in the preparation of hydrogen chloride and nitric acid?
(b) What property of hydrogen chloride is demonstrated when it is collected by the downward delivery (upward displacement)?
Ans. (a) Conc. sulphuric acid is the least volatile acid and hence does not distil over while preparing HCl or HNO3.
(b) It shows HCl is heavier than air.
14. Write balanced chemical equations for the following reactions:
(a) Zinc and dilute hydrochloric acid
(b) Calcium bicarbonate and dilute hydrochloric acid
Ans. (a) Zn + 2HCl (dil.) → ZnCl2 + H2(g)
(b) Ca(HCO3)2 + 2HCl (dil.) → CaCl2 + 2H2O + 2CO2 (g)
15. (a) Dilute nitric acid is generally considered a typical acid except for its reaction with metals. In what way is dilute nitric acid different from other acids when it reacts with metals?
(b) Write the equation for the reaction of dilute nitric acid with copper.
Ans. (a) Generally, the dilute acids react with the active metals to form their respective salts and hydrogen gas. However, dilute nitric acid is a powerful oxidising agent. It reacts with the nascent hydrogen liberated during its action with metals and hence oxidises it to form water. Dilute nitric acid by itself is reduced to nitric oxide gas.
(b) 3Cu(s) + 8HNO3(aq) 3Cu(NO3)2(aq) + 2NO(g) + 4H2O(I)
16. (a) Which feature of ammonia molecule leads to the formation of ammonium ion when ammonia dissolves in water.
(b) Name the other ion formed when ammonia dissolves in water.
Ans. (a) It is the presence of lone pair of electrons in the molecule of ammonia gas which leads to the formation of ammonium ions, when dissolved in water.
(b) The other ion formed is hydroxyl [OH] ion.
17. Mention the property of conc. H2SO4 exhibited in each of the following reactions with:
(a) sugar
(b) metallic chloride
Ans. (a) Dehydration of organic compound
(b) As a non-volatile acid
18. A, B, C and D summarize the properties of sulphuric acid depending on whether it is dilute or concentrated.
A = Typical acid property
B = Non Volatile acid
C = Oxidizing agent
D = Dehydrating agent
Choose the property (A, B, C or D) depending on which is relevant to each of the following:
(a) Preparation of hydrogen chloride gas.
(b) Preparation of copper sulphate from copper oxide.
(c) Action of conc. sulphuric acid on sulphur.
Ans. (a) B (Non volatile acid)
(b) A (Typical acid property)
(c) C (Oxidising agent)
19. Fill in the blanks with the correct words from the brackets:
Alkenes are the (i) __________ (analogous/homologous) series of (ii) ____________ (saturated/un-saturated) hydrocarbons. They differ from alkanes due to the presence of (iii) _________ (double/single) bonds. Alkenes mainly undergo (iv) ___________ (addition/substitution) reactions.
Ans. (i) homologous
(ii) unsaturated
(iii) double
(iv) addition
20. Draw the structural formulae of the two isomers of butane. Give the correct IUPAC name of each isomer.
Ans.

3 Marks Questions
1. An element with atomic number 18 is a noble gas. Into which families will you place elements with atomic numbers 17 and 19 and why?
Ans. (i) The electronic configuration of element with atomic number 17 is (2, 8, 7). As the element has 7 electrons in its valence shell, it belongs to the halogen family.
(ii) The electronic configuration of element with atomic number 19 is (2, 8, 8, 1). As the element has 1 electron in its valence shell, it belongs to the alkali metal family.
2. (a) (i) Which period in the Periodic Table is the shortest?
(ii) Name all the elements present in this period.
(b) (i) Which period in the Periodic Table is the longest and complete?
(ii) How many elements are present in it?
Ans. (a) (i) The first period is the shortest.
(ii) The elements of the first period are hydrogen and helium.
(b) (i) The sixth period is complete and longest.
(ii) It has 32 elements.
3. Metallic properties of the elements change to non-metallic properties as one moves from left to right in a period of the periodic table. Explain.
Ans. If an element donates its valence electrons with ease so as to form positively charged ions, it is said to be a metallic element. Conversely, if an element accepts electrons in its valence shell so as to form negatively charged ions, the element is said to be non-metallic.
On progressing in a period from left to right, there is a gradual increase in the nuclear charge which results in the decrease in volume of elements. This results in more tightly bound electrons in the valence shell and makes it difficult for the atoms of elements to donate electrons. Thus, the character of elements gradually changes from metallic to non-metallic.
4. State why are noble gases unreactive while atoms of elements other than noble gas are chemically reactive.
Ans. It has been established that if any element has two electrons in all, and these electrons are in the valence shell (duplet structure) or eight electrons in its valence shell, then the element is in the minimum state of energy. Such an element cannot donate/accept/share electrons with other elements and hence does not form a chemical bond. As noble gases have duplet or octet structure, therefore, they are chemically inactive. However, all other elements do not have structure like that of noble gases. They can donate/accept/share electrons from their valence shell
and hence are chemically active.
5. What is meant by the term “electrovalency”? State why sodium (at. no. 11] has electrovalency +1 and chlorine (at. no. 17) has electrovalency of -1.
Ans. Electrovalency: The number of electrons donated (lost) or accepted (gained) by an atom of an element, from its valence shell, such that the ion formed (residual particle) has an electronic configuration of the nearest noble gas is called electrovalency of the element.
If the electrons are donated, then the electrovalency is said to be positive. If the electrons are accepted, then the electrovalency is said to be negative.
Sodium atom [at. no. 11] has 11 protons in its nucleus and its electronic configuration is (2, 8, 1). The sodium atom donates one electron from its valence shell to have the electronic configuration of the nearest noble gas neon. However, in doing so, it has 11 protons in its nucleus and 10 electrons. Thus, the residual atom (sodium ion) has a unit positive charge or has electrovalency + 1.
Chlorine atom (at. no. 17) has 17 protons in its nucleus and its electronic configuration is [2, 8, 7]. The chlorine atom accepts one electron in its valence shell to have the electronic configuration of the nearest noble gas argon.
6. State two chemical properties each with equations for (i) a solution containing H(aq) ions (ii) a solution containing OH ions.
Ans:

7. A compound gave a following data:
C = 57.82%, O= 38.58% and the rest hydrogen. Its relative molecular mass is 166.
Find its empirical formula and molecular formula.
[C = 12, O= 16, H = 1]
Ans:

11. Answer the following questions about electroplating a copper wire with silver.
(i) What ions must be present in the electrolyte?
(ii) of what substance must the anode be made?
(iii) What should be made cathode?
Ans. (i) Silver ions must be present in electrolyte.
(ii) The anode should be made from silver.
(iii) The article to be electroplated should be made the cathode.
12. Three different electrolytic cells A, B and C are connected in separate circuits. Electrolytic cell A contains sodium chloride solution. When the circuit is completed a bulb in the circuit glows brightly. Electrolytic cell B contains acetic acid solution and in this case the bulb in the circuit glows dimly. The electrolytic cell C contains sugar solution and the bulb does not glow. Give a reason for each of these observations.
Ans. Electrolytic cell A has completely ionised sodium chloride solution. Thus, the ions can easily migrate to oppositely charged poles and hence the bulb glows brightly. To conclude sodium chloride solution is a strong electrolyte. Electrolytic cell B has a weak electrolyte as only 5% of the acetic acid molecules ionise. Thus, a weak current flows through it and hence the bulb glows dimly. Electrolytic cell C has a non-electrolytic. Sugar molecules do not ionise and hence no current flows through it. Thus, the bulb does not glow.
13. (a) Name the chief ore of aluminium.
(b) Name the process used to concentrate the above mentioned ore.
(c) Why is alumina added to cryolite in the electrolytic reduction of aluminium?
Ans. (a) Bauxite
(b) Hall’s process or Baeyer’s process.
(c) The cryolite lowers the melting point of alumina and makes it a better conductor.
14. When concentrated hydrochloric acid is treated with potassium permanganate crystals a greenish yellow gas is given out.
(a) Name the gas evolved.
(b) Write a fully balanced chemical equation for the reaction.
(c) Is hydrochloric acid acting as oxidising agent or reducing agent?
Ans. (a) The gas evolved is chlorine gas.
(b) 2KMnO4 + 16HC1 → 2KCl + 2MnCl2 + 8H2O + 5Cl2
(c) Hydrochloric acid acts as a reducing agent.
15. Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. Answer the questions that follow based on this reaction:
(a) Give the balanced chemical equation for the reaction with suitable condition(s) if any.
(b) Why is concentrated sulphuric acid used instead of concentrated nitric acid?
(c) How is the gas collected?
Ans.
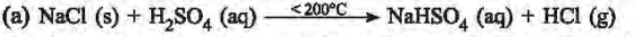
(b) Conc. sulphuric acid is used since it is non-volatile and has a high boiling point. So it displaces the volatile hydrogen chloride from the salt sodium chloride.
(c) Dry hydrogen chloride gas is collected by the upward displacement of air.
16. (a) Write an equation for the formation of ammonia gas by the action of water on magnesium nitride.
(b) How is ammonia collected?
(c) Why is ammonia not collected over water?
Ans. (a) Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3 (g)
(b) Ammonia is collected by the downward displacement of air.
(c) It is because ammonia gas is extremely soluble in water.
17. The following questions are based on the preparation of ammonia gas in the laboratory :
(a) Explain why ammonium nitrate is not used in the preparation of ammonia.
(b) Name the compound normally used as a drying agent during the process.
(c) How is ammonia gas collected?
Ans. (a) Ammonium nitrate on heating decomposes explosively to form nitrous oxide gas and water.
(b) Quick lime is used for drying ammonia gas.
(c) Ammonia is collected by the downward displacement of air from the gas jar.
18. (a) Name the process used for the large scale manufacture of sulphuric acid.
(b) Which property of sulphuric acid accounts for its use as a dehydrating agent?
(c) Concentrated sulphuric acid is both, an oxidising agent and a non-volatile acid. Write one equation each to illustrate the above mentioned properties of sulphuric acid.
Ans. (a) Contact Process is used in the manufacture of sulphuric acid.
(b) The property is called dehydrating property, as it has a strong affinity for water.
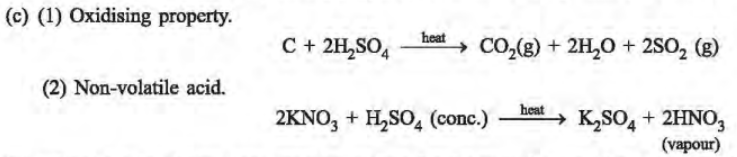
19. (a) With the help of equations, give an outline for the manufacture of sulphuric acid by the contact process.
(b) What property of sulphuric acid is shown by the reaction of concentrated sulphuric acid when heated with
(i) Potassium nitrate (ii) Carbon
Ans.

20. Write the equations for the following laboratory preparations :
(a) Ethane from sodium propionate.
(b) Ethene from iodoethane.
(c) Ethyne from calcium carbide.
Ans:

5 Marks Questions
1. An atom A (at. no 11) and an atom B (atomic number 17) belong to 3rd period of long form of periodic table.
(i) Name the atom(s) which is/are more metallic amongst A and B.
(ii) The (A) and (B) belong to the which families of elements?
(iii) Name the type of bond formed between A and B is.
(iv) State the solubility of the compound A and B.
(v) What is position of A and B in long form of periodic table?
Ans. (i) A is more metallic
(ii) A belongs to alkali metals and B belongs to halogens
(iii) Ionic
(iv) Highly soluble in water
(v) A belongs to group 1 and B to group 17
2. Amongst E, F, G and H elements, of atomic number 4, 9, 15 and 16 respectively.
(i) Name the element of the group 17.
(ii) In the given above the elements which has three electron shells?
(iii) In the given above name the element with highest electronegativity.
(iv) In the given above which is a metal?
(v) In the given above which element is a non-metal?
Ans. (i) Element F
(ii) G and H
(iii) Element F
(iv) Element E
(v) F, G and H
3. Elements E and F have atomic number 6 and 9 respectively.
(i) Name formula of their chloride.
(ii) Name the bond having compound E and F.
(iii) What is the expected M.P. of the compound of E and F?
(iv) What is nature of the conductivity of the compound of E and F with respect to electricity?
(v) What is expected solubility of compound E and F in water?
Ans. (i) EF4
(ii) Covalent bonds only
(iv) Non-conductor of electricity
(iii) Low
(v) Insoluble in water
4. For each of the conversions A, B, C, D and E in the scheme below, state briefly in words, how the conversions can be carried out.

Ans:

5. (a) Why does molten sodium chloride decompose on the passage of electric current?
(b) (i) Name two metals normally manufactured by the electrolysis of fused compounds.
(ii) Name two compounds used in (b) (i) above.
(iii) For one of the above metals, explain the reaction taking place at the cathode.
Ans. (a) On the passage of electric current through molten sodium chloride, sodium ions migrate to the cathode and chloride ions migrate to the anode.
At the cathode, sodium ions gain electrons and at the anode, chloride ions lose electrons to form sodium and chlorine atoms.

6. A metal article is to be electroplated with silver. The electrolyte selected is sodium argentocyanide.
(i) What kind of salt is sodium argentocyanide?
(ii) Why is it preferred to silver nitrate as an electrolyte?
(iii) State one condition to ensure that the deposit is smooth, firm and long lasting.
(iv) Write the reaction taking place at the cathode.
(v) Write the reaction taking place at the anode.
Ans. (i) Sodium argentocyanide is a complex salt.
(ii) Sodium argentocyanide is preferred to silver nitrate as it does not hydrolyse in aqueous solution.
(iii) The metal to be electroplated should be absolutely free from oxides of metals, grease, etc., and low current density should be used.

7. The following is a sketch of an electrolytic cell used in the extraction of aluminium:

(i) What is the substance of which the electrodes A and B are made?
(ii) At which electrode (A or B) is aluminium formed?
(iii) What are the two aluminium compounds in the electrolyte C?
(iv) Why is it necessary for electrode B to be continuously replaced?
Ans. (i) A is gas carbon. B is carbon rod.
(ii) Aluminium is formed on electrode A.
(iii) Fused alumina (Al2O3) and cryolite [Na3AIF6].
(iv) The electrode B is continuously consumed due to the liberation of oxygen, which oxidises it to carbon dioxide.
8. A colourless gas G fumes strongly in the air. The gas gives dense white fumes when a glass rod dipped in ammonia solution is held near the gas. Answer the following questions:
(i) Name the gas G.
(ii) Name two chemicals used in the preparation of the gas G.
(iii) Write the chemical equations for the reaction of the chemicals named in (ii) when :
(a) the reaction mixture is not heated.
(b) the reaction mixture is heated above 200°C.
(iv) Why does the gas G fume strongly in air ?
(v) Why does the gas G form dense white fumes with ammonium hydroxide?
Ans. (i) The gas G is hydrogen chloride gas.
(ii) The chemicals are (i) sodium chloride (ii) conc. sulphuric acid

(iv) It is because the HCl gas is extremely soluble in water. Thus, the gas dissolves in water vapour present in the air to form tiny droplets of hydrochloric acid, which appear in the form of fumes.
(v) The HCl gas reacts with vapours of ammonium hydroxide to form very fine solid particles of ammonium hydroxide which are white in colour. These white particles of solid ammonium hydroxide appear in the form of white fumes.
9. (i) When nitric acid is prepared by the action of concentrated sulphuric acid and potassium nitrate, what is the special feature of the apparatus used?
(ii) Write a balanced equation for reaction in 6(i)
(iii) What type of reaction takes place when potassium nitrate is prepared from potassium hydroxide and nitric acid?
(iv) Which gas is produced when potassium nitrate is heated? Write an equation for the reaction.
Ans.

10.

(i) Name a catalyst used in converting sulphur dioxide to sulphur trioxide as shown in step C.
(ii) In the manufacture of sulphuric acid, sulphuric trioxide is not directly absorbed in water. Instead a two-step procedure is followed. Write two equations involved in the step D.
(iii) What type of a chemical substance will liberate sulphur dioxide from sodium sulphite in step E?
(iv) Write an equation for step F, in which sulphur dioxide is converted into sodium sulphite.
Ans. (i) The catalyst used for converting sulphur dioxide to sulphur trioxide is vanadium pentoxide at 450°C.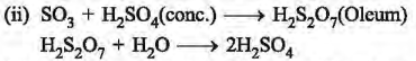
(iii) Mineral acids (HCl, H2SO4) in dilute form will react with sodium sulphite with the liberation of sulphur dioxide gas.
Comments are closed