Play and Listen here
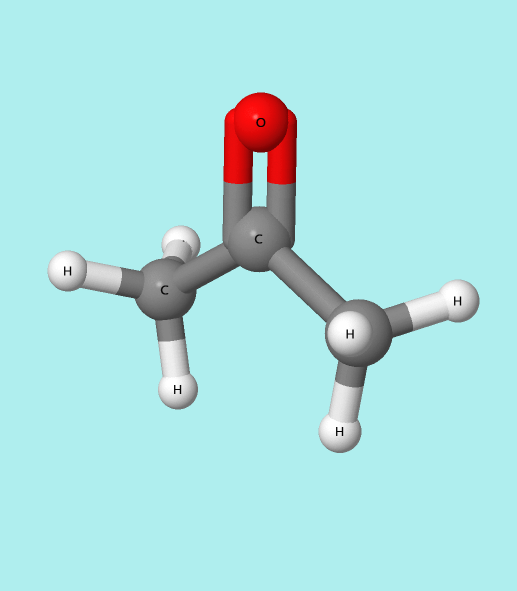
The functional group present in propanone is a ketone.
Here’s a breakdown of what that means,
- Imagine a carbon chain like a backbone. In a ketone, a carbonyl group sits in the middle of the chain, like a pendant.
- The carbonyl group consists of a carbon atom double-bonded to an oxygen atom (C=O). This double bond makes the ketone reactive and gives it unique properties. Here ” = ” is double bond.

Think of it like this:
- If the carbonyl group were a superhero, the carbon atom would be the hero, and the oxygen atom would be its sidekick. They’re always together, and they have a special bond that makes them powerful.
Another example of a molecule with a ketone group is acetone, which is used as a solvent in nail polish remover.
Here’s how the ketone group affects propanone:
- It makes propanone a polar molecule, meaning it has a slightly positive end and a slightly negative end. This polarity allows it to dissolve in water and other polar solvents.
- It also allows propanone to participate in reactions called hydrogen bonds, which are important in many biological processes.
In summary, propanone contains a ketone group (C=O) that plays a role in its chemical properties.


Leave a Reply