Indicators
An indicator is a dye which changes color when it is added to an Acid or a Base. Some major types of acid – base indicators are as follows –
- Natural indicator
- Synthetic indicator
- Olfactory indicator
Natural indicator
Those indicators that are extracted from plants are called natural indicators. Some natural indicators are –
- Litmus – It is a Purple Dye extracted from a type of plant called
(i) An acid turns blue litmus to
(ii) A base (or alkali) turns red litmus to blue - Turmeric – It is a Yellow Dye present in Haldi.
(i) It turns Red in an Acid
(ii) No change in base
Synthetic indicator
Those indicators that are synthesized in the laboratory are called synthetic indicators. Some Synthetic indicators are –
- Methyl orange – It is an orange coloured
(i) It turns red in an
(ii) It turns yellow in - Phenolphthalein – It is a colourless
(i) No change in an
(ii) It turns pink in base.
Olfactory indicator
The substances whose smell ( or Odour ) changes in acidic or Basic solutions are called Olfactory indicators. Some Olfactory indicators are –
Onion and Vanilla extracts
(i) An acid does not destroy Onion and Vanilla extracts smell.
(ii) A base destroys Onion and Vanilla extracts smell.
Acids
An acid is a substance which dissociates (or Ionize) on dissolving in water to produce hydrogen ions ( H+ (aq) ions).
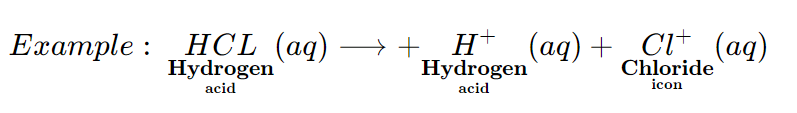
The presence of hydrogen ions (H+ (aq) ions) in hydrochloric acid solution in water, makes it behave like an acid.
The hydrogen ions (H+ (aq) ions) do not exist in water as H+ (aq.) ions. The hydrogen ions (H+ (aq) ions) attach themselves to the polar water molecule to form hydronium ions (H3O+ (aq) ions).
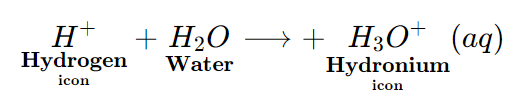
The presence of hydrogen ions [ H+ (aq) ions] in the aqueous solution of an acid, gives an acid its acidic properties.
Strong acids – An acid which dissociates to a large extent to produce a large number of Hydrogen ions (H+) in water are called Strong Acids.
Example – Mineral acids like Hydrochloric acid (HCl), Sulphuric acid (H2SO4), etc.
Weak acids – An acid which dissociates to a small extent to produce a smaller number of Hydrogen ions (H+) in water are called Weak Acids.
Example – Mineral acids like Carbonic acid (H2CO3), Organic acids like Acetic acid (CH3COOH), etc.
Properties of acids
- Acids have a Sour taste.
- Acids turn blue litmus Red.
- Acid solutions conduct electricity (i.e. They are electrolytes)
- Acids react with metals to form hydrogen gas.
Metal + Dilute Acid ⟶ Metal Salt + Hydrogen
Zinc metal reacts with dilute Sulphuric Acid to form Zinc Sulphate Salt and liberate hydrogen gas
Zn(s) + H2SO4(Dil.) ⟶ ZnSO4(aq) + H2(g) - Acids react with metal oxides to form Salt and water.
Metal oxides + Dilute Acids ⟶ Salt + Water CuO + 2HCl ⟶ CuCl2 + H2O
Like acids react with bases to form salt and water, similarly metallic oxides also react with acids to give salt and water, hence, metallic oxides are said to be Basic oxides. - Acids react with metal carbonates and Metal Hydrogen Carbonates to form Water and Carbon Dioxide gas.
Metal Carbonates + Acid ⟶ Metal Salt + Water + Carbon dioxide Calcium Carbonate reacts with Hydrochloric Acid to form Calcium Chloride salt, water and carbon dioxide gas.
CaCO3(s) + 2HCl(aq) ⟶ CaCl2(aq) + H2O(l) + CO2(g)
Metal Hydrogen Carbonates + Acid⟶Metal Salt + Water + Carbon dioxide Sodium Bicarbonate reacts with Sulphuric Acid to form Sodium Sulphate Salt and Water and Carbon Dioxide gas.
2NaHCO3(s) + H2SO4(aq) ⟶ Na2SO4(aq) + 2H2O(l) + 2CO2(g) - Acids react with Bases(or Alkalis) to form Salt and water.
- Acids have a Corrosive Nature.
Bases
An Base is a substance which dissociates (or Ionize) on dissolving in water to produce hydroxide ions (OHー (aq) ions).
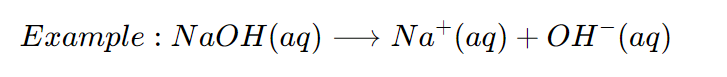
The presence of hydrogen ions (OHー (aq) ions) in Sodium hydroxide solution in water, makes it behave like a base.
Strong bases – A base which dissociates to a large extent to produce a large number of Hydroxyl ions (OHー) in water are called Strong Bases.
Example – Sodium hydroxide (NaOH), Potassium hydroxide (KOH), etc.
Weak bases – A base which dissociates to a small extent to produce a smaller number of Hydroxyl ions (OHー) in water are called Weak Bases.
Example – Calcium hydroxide (Ca(OH)2), Zinc hydroxide (Zn(OH)2), etc.
Bases generate hydroxide (OHー) ions in water. Bases which are soluble in water are called alkalis.
Properties of bases
- Bases have a Bitter ( Kadwa ) taste .
- Bases are Soapy to touch.
- Bases turn Red litmus Blue .
- Base solutions Conduct electricity (they are electrolytes).
- Base reacts with some metals to form Metal Salts and Hydrogen gas.
Metal + Base ⟶ Metal Salt + Hydrogen
Sodium Hydroxide reacts with Zinc to form Sodium Zincate Salt and hydrogen gas.
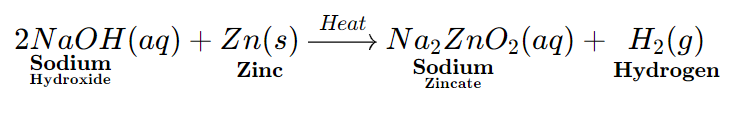
The Metal is present in the salt formed as a part of the Negative ion (or Anion)
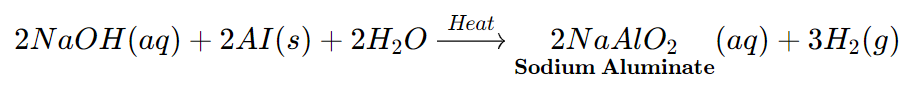
*All the Metals do not react with Bases to form Salt and Hydrogen gas.
6. Bases react with Acids to form Salt and Water.
Basicity of acids-
The number of Hydrogen ions produced by an acid on ionisation in water, is called as its basicity.
its basicity.
H2SO2(aq)→2H+(aq)+SO42-(aq)
Acidity of a base
Acidity of a base is defined as the number of Hydroxyl groups present in a moecule ofa base
Ca(OH)2(aq)→Ca++(aq)+2OH–(aq)
Neutralisation reaction
When aqueous solutions of an Acid and a Base combine to form Salt and water, the chemical reaction is called a Neutralization reaction. Here, the hydrogen ions of the Acid (H+) and the Hydroxyl Ions of the Base (OH−) combine with each other to form water, whereas, the Metal part of the Base combines with the Non-Metal part of the Acid to form Salt.
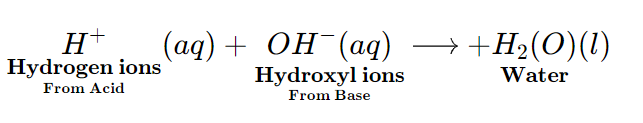
precaution While diluting of an acids-
Dissolving an acid or a base in water is a highly exothermic process. The acid must always be applied gently to the water while Strring constantly. When water is added to a concentrated acid, the heat produced may cause the liquid to splash out, causing burns, excessive surface heat may potentially cause the glass container to break.
pH scale
It may be described as a number that must be multiplied by a negative power of ten to indicate the concentration of hydrogen ions in the solution.
[H+] = 10-pH
The concentration of hydrogen ions (H+) is expressed as moles/litre and is written as [H+].
[H+] = 10-1 ; Then pH = 1
[H+] = 10-4 ; Then pH = 4
pH may also be defined as negative logarithm (base 10) of the hydrogen ion concentration in moles per litre. Mathematically
pH = -log10[H+]
OR
pH = -log10[H3O+]

Role of pH in Everyday Life
- In Biology;
- In Humans and Animals: pH range : 7.0 – 7.8. Examples : Human Blood , Tears , Saliva has pH = 7.4
- In Plants: For healthy growth, pH should be Neutral
- In Digestive System: pH range ⟶ Acidic
- In Tooth Decay : pH range ⟶ Below 5 Enamel Dissolves, leading to tooth decay
- In Self Defense -Through chemical warfare. Examples : Sting of Honey Bees , Yellow Ants are slightly acidic.
- In regaining shine of tarnished Copper vessel : Tarnishing of Copper vessel happens due to the formation CuO which is Basic in nature. It can be removed by using lemon juice which is acidic in nature.
Salts
Salt is a compound formed by the complete neutralization of an Acid by a Base or of a Base by an Acid. It is an ionic compound which contains a Positive ion (a Cation) other than Hydrogen ion and a Negative ion (an Anion) other than Hydroxyl ion.
- Chlor – alkali process – Chemical formula – NaOH
Chemical name – Sodium hydroxide
Common name – Caustic soda
When electricity passes through aqueous sodium chloride (called brine), it decomposes to generate sodium hydroxide. As a result of the products formed–chlor for chlorine and alkali for sodium hydroxide. This process is known as the chlor-alkali process.
At Anode: 2Cl–(aq)– 2e– → Cl2↑
At Cathode: Na+(aq)+ e– → Na(s)
Solution: Na(s)+ OH–(aq)→ NaOH(aq)
The applications of the products produced by this method –
- Hydrogen gas is utilized as a fuel, in the production of ammonia, and in the hydrogenation of oils.
- Chlorine gas is employed as a disinfectant in producing the PVC and CFC.
- Sodium hydroxide is used to degrease metals, as well as in the production of soaps, detergents, and paper.

When Chlorine gas is passed through the Slaked lime (Calcium Hydroxide), Bleaching powder is formed.
Ca(OH)2 + Cl2 ⟶ CaOCl2 + H2O
Uses –
- To disinfect drinking water, bleaching powder is used.
- Chloroform (CHCl3), an anaesthetics, is manufactured using bleaching powder.
- In the paper industry, bleaching powder is also used.
- Wool shrinkage is prevented by the application of bleaching powder.
- In industry, bleaching powder is used as an oxidizing agent.
- Baking soda
Chemical formula – NaHCO3
Chemical name – Sodium Hydrogen Carbonate
Common name – Baking soda

Structural formula
When Carbon Dioxide and Ammonia gas are simultaneously passed through Sodium Chloride solution in water (Brine)-
NaCl + H2O + CO2 + NH3 ⟶ NaHCO3 + NH4Cl
Sodium Bicarbonate ( Baking Soda ) and Ammonium Chloride is formed.
Uses –
- For making Baking Powder :
Baking powder is a mixture of Sodium Hydrogen Carbonate and an edible Acid like Tartaric Acid. When Baking Powder is Heated or Mixed with Water, The following Reaction Takes place
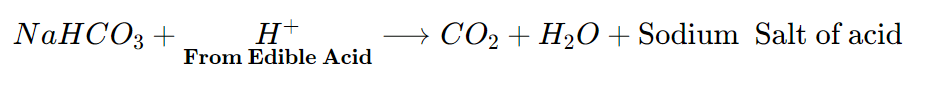
- The CO2 produced during the reaction causes bread or cake to rise making them soft and spongy.
- It is used as an ingredient in Antacids. Being Alkaline, it Neutralizes excess acid in the stomach, and provides relief.
- It is also used in Soda-Acid fire- Extinguishers.
- Washing soda
Chemical formula – Na2CO3.10H2O
Chemical name – Sodium Carbonate
Common name – Washing soda


Anhydrous (or dried) form of washing soda is called Soda ash.
Uses
- Industrial soap, glass, and paper production.
- For the treatment of hard water produced by chlorides and sulphates.
- In the form of detergents, as a cleaning agent.
- In the production of sodium compounds such as Borax (Na2B4O7.10H2O, Hydrated Sodium Borate).
- Plaster of paris
Chemical formula – CaSO4.½ H2O
Chemical name – Calcium Sulphate HemiHydrate
Common name – Plaster Of Paris (P.O.P.)

Plaster of Paris derived its name from the fact that it was made from gypsum (CaSO4.2H2O) which was found mainly in Paris.
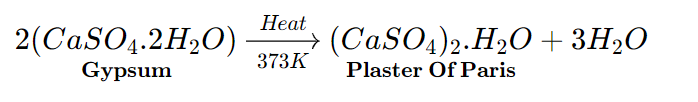
The gypsum should be heated carefully under regulated conditions because if it is heated over 100°C, anhydrous Calcium Sulphate (CaSO4) is created, which does not set like Plaster of Paris when water is added.
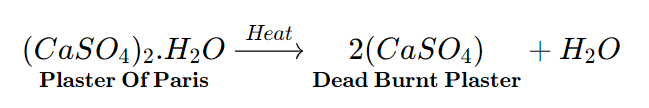
Uses
- In the creation of toys, idols, and statue castings
- When making denture casts
- In bone-setting, or the repair of cracked limbs and bone joints.
- Sealing air holes in laboratory equipment during investigations
- To make blackboard chalk, ornamental materials, POP ceilings,
cosmetics, and so forth.
- Acids do not show acidic behavior in absence of water
The presence of hydrogen ions, H+(aq) ions, in acids causes their acidic behaviour. Only in the presence of water do acids create hydrogen ions. In the absence of water, the chemical does not generate hydrogen ions and so does not exhibit acidic behaviour. Because dry HCl gas contains no hydrogen ions, it does not exhibit acidic behaviour. As a result, dry HCl gas has no effect on the colour of dry blue litmus paper. - When concentrated acid is added to water, heat is gradually released and
absorbed by the enormous volume of water. When water is added to concentrated acid, a tremendous amount of heat is immediately created. This heat converts part of the water into steam, resulting in acid splashing on the face or clothes and severe burns on the skin.
What are Acid Bases and Salts?


Families of Salts
- Sodium Salts — NaCl, NaNO3, Na2SO4, Na2CO3, CH3COONa etc.
- Potassium Salts — KCl, KNO3, K2SO4, KBr, K2CO3
- Ammonium Salts— NH4Cl, NH4NO3, NH4Br
- Magnesium Salts— MgCl2, MgSO4, MgCO3
- Calcium Salts— CaCl2, Ca(COO)2, etc.
Chloride Salts
| Salts and their Bases | |||
| Formula | Name of Salt | Base Involved | |
| NaCl | Sodium chloride | NaOH | |
| KCl | Potassium chloride | KOH | |
| NH4Cl | Ammonium chloride | NH4OH | |
| BaCl2 | Barium chloride | Ba (OH)2 | |
| MgCl2 | Magnesium chloride | Mg(OH)2 | |
Nitrate SaltsF
| Salts and their Bases | |||
| Formula | Name of Salt | Base Involved | |
| NaNO3 | Sodium nitrate | NaOH | |
| KNO4 | Potassium nitrate | KOH | |
| Ca(NO3)2 | Calcium nitrate | Ca(OH)2 | |
Sulphate Salts
| Salts and their Bases | |||
| Formula | Name of Salt | Base Involved | |
| Na2NO3 | Sodium sulphate | NaOH | |
| K2SO4 | Potassium sulphate | KOH | |
| MgSO4 | Magnesium sulphate | Mg(OH)2 | |
Carbonate Salts
| Salts and their Bases | |||
| Formula | Name of Salt | Base Involved | |
| Na2NO3 | Sodium Carbonate | NaOH | |
| K2CO3 | Potassium Carbonate | KOH | |
| CaCO3 | Calcium Carbonate | Ca(OH)2 | |
| Keywords | |
| 1. Acid: An acid is any hydrogen-containing substance that is capable of donating a proton (hydrogen ion) to another substance. They are known to turn blue litmus paper to red. | 11. Indicators: Indicators are weak acids or weak bases that show a change in colour as the concentration of Hydrogen ions in a solution changes or the pH of a solution changes. The indicators dissociate slightly in the water to form ions. Some examples of indicators are Litmus, turmeric, phenolphthalein, etc. |
| 2. Base: A base is a molecule which is able to accept a hydrogen ion from an acid. They are known to turn red litmus paper to blue. | 12. pH: pH measures the strength of acid and bases. pH stands for the potential of Hydrogen, and is approximately the negative of the base 10 log of molar concentration of H+ ions. |
| 3. Ion: An atom or molecule with a net electric charge due to the loss or gain of one or more electrons. | 13. Universal Indicator: Universal indicator is defined as the mixture of different indicators that gives different colours at different pH levels of the entire scale. It helps to interpret the acidic or basic nature of a substance. It exhibits several colour changes over a pH value range from 0 to 14 to indicate the acidity or alkalinity of solutions, where pH value 7 indicates neutral, pH value below 7 indicates acidity and pH value above 7 indicates alkalinity or basicity of a solution. |
| 4. Ionisation: The process of forming ions in aqueous solution is called ionisation. | 14. Hydrogenation: A chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. A chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. |
| 5. Salt: The compound formed by reaction of an acid with a base, e.g., NaCl. | 15. Antacid: The substance which neutralizes acidity (especially in the stomach). The substance which neutralizes acidity (especially in the stomach). |
| 6. Strong acid: An acid which dissociate completely when dissolved in water furnishing is called as H+ ions strong acid. | 16. Aqua regia: It is a mixture of concentrated hydrochloric acid (HCl) and concentrated nitric acid (HNO3) at a ratio of either 3 : 1. |
| 7. Strong base: A base which dissociates completely in aqueous solution furnishing OH– ions is called as strong base. | 17. Enamel: This tough shell is the hardest tissue in the human body and it covers the crown which is the part of the tooth that is visible outside of the gums. |
| 8. Weak acid: A weak acid is one which does not ionise fully, when it is dissolved in water. | 18. Hard water: Water that contains mineral salts (as calcium and magnesium ions) which limits the formation of lather with soap. |
| 9. Weak base: A weak base is one which does not ionise fully, when it is dissolved in water. | 19. Amphoteric substance: The substance having property of both acid as well as base. The substance having property of both acid as well as base. |
| 10. Alkali: An alkali is a substance that produces OH– ions in water. | |
check more…


Leave a Reply