Play & Listen Here
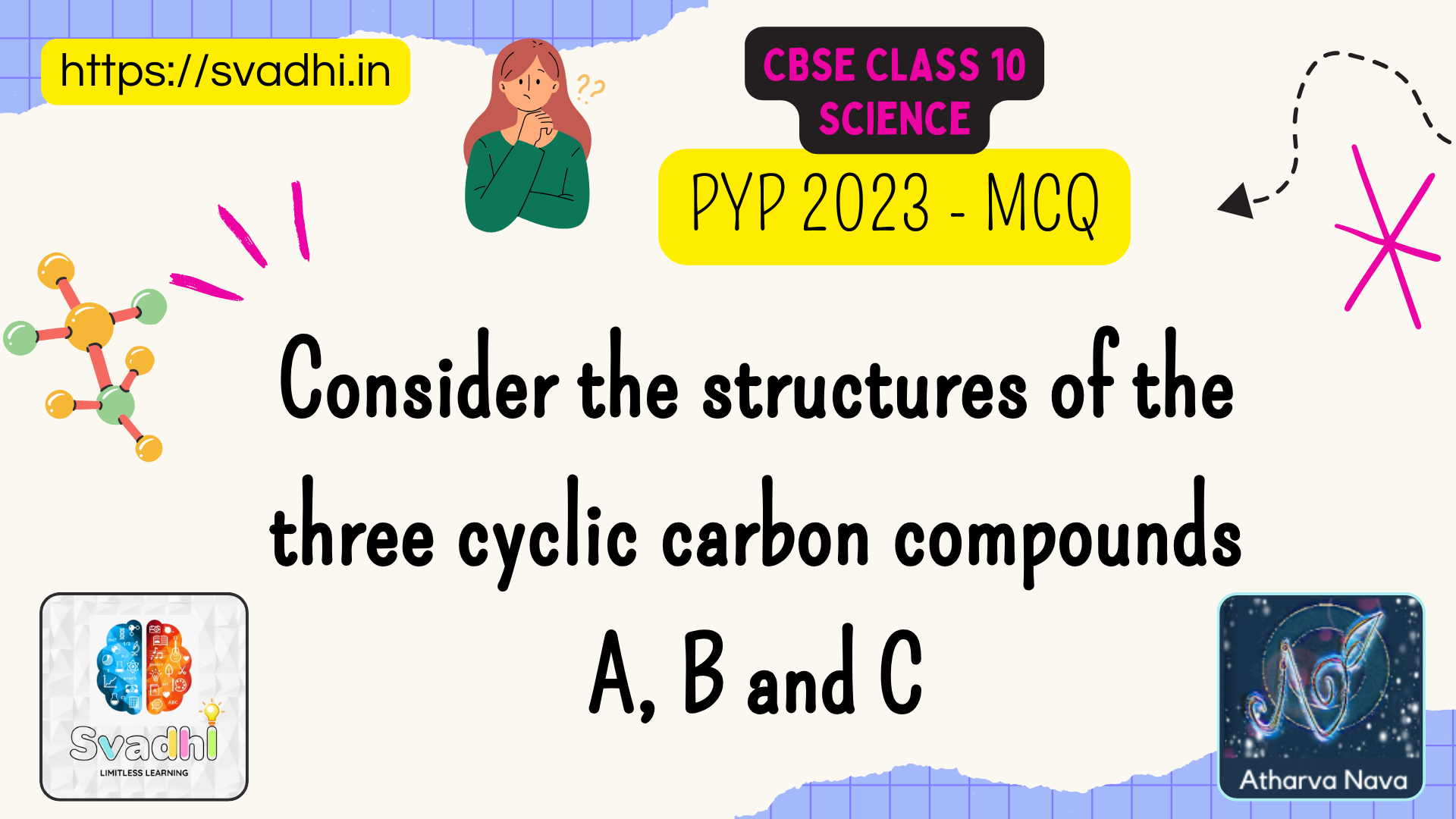
A, B and C given below and select the correct option from the following:

- A and C are isomers of hexane and B is benzene.
- A is an isomer of hexane, B is benzene and C is an isomer of hexene.
- A is a saturated cyclic hydrocarbon and B and C are unsaturated cyclic hydrocarbons.
- A is cyclohexane and B and C are the isomers of benzene.
Analysis of the figures:
The three structures shown in the image are all cyclic hydrocarbons, meaning they consist of carbon atoms arranged in a closed ring. Let’s analyze them one by one:
Figure A:
- This molecule has six carbon atoms and twelve hydrogen atoms.
- All the carbon atoms are connected to each other by single bonds, and each carbon atom is also bonded to two hydrogen atoms.
- This structure satisfies the definition of an alkane, which is a hydrocarbon that only contains single bonds between the carbon atoms.
- Additionally, all the carbon atoms are sp3 hybridized, meaning they have four bonding orbitals.
- Because each carbon atom bonds with four other atoms, all the bonds in this molecule are single bonds, and the molecule does not contain any rings of atoms.
- Therefore, molecule A is cyclohexane.


Figure B:
- This molecule has six carbon atoms and six hydrogen atoms.
- It has a ring structure with alternating single and double bonds between the carbon atoms.
- This structure satisfies the definition of an aromatic hydrocarbon, which is a cyclic hydrocarbon that has alternating single and double bonds between the carbon atoms.
- Additionally, all the carbon atoms are sp2 hybridized, meaning they have three bonding orbitals and one non-bonding orbital.
- The delocalized electrons in the p orbitals above and below the ring contribute to the stability of the molecule.
- Therefore, molecule B is benzene.


Figure C:
- This molecule has six carbon atoms and eight hydrogen atoms.
- It has a ring structure with two double bonds between two carbon atoms.
- This structure satisfies the definition of an alkene, which is a hydrocarbon that contains one or more carbon-carbon double bonds.
- Additionally, all the carbon atoms are sp3 hybridized, except for the two carbons that are bonded to each other by a double bond, which are sp2 hybridized.
- Therefore, molecule C is an isomer of hexene.


Key points:
- Saturated hydrocarbons have only single bonds between the carbon atoms.
- Unsaturated hydrocarbons have one or more double or triple bonds between the carbon atoms.
- Isomers are molecules that have the same chemical formula but different structures.
Conclusion:
Based on the analysis above, we can see that:
- Molecule A (cyclohexane) is a saturated cyclic hydrocarbon, meaning it only contains single bonds between the carbon atoms.
- Molecule B (benzene) and molecule C (isomer of hexene) are unsaturated cyclic hydrocarbons, meaning they contain at least one carbon-carbon double bond.
Therefore, option (c) is the correct answer: “A is a saturated cyclic hydrocarbon and B and C are unsaturated cyclic hydrocarbons.“
1,753 Responses
Sweet blog! I found it while browsing on Yahoo News. Do you have any
suggestions on how to get listed in Yahoo News?
I’ve been trying for a while but I never seem to get there!
Thank you
comprar medicamentos en Italia lyfis Duivendrecht
Compra medicamentos sin receta médica
medicijnen: effectieve pijnverlichting zonder gedoe egis Salamanca leki zalecane przez
lekarzy
приснилась чужая кровь к чему это сонник уговариваю я
посмотреть расклады на таро
какая ты прокладка по знаку
зодиака знак зодиака рыбы с какого по какое февраля
dove trovare farmaci in Italia Qualigen Limburg Acheter
de la médicaments en toute sécurité et confidentialité
Having read this I believed it was rather informative. I appreciate you spending some time
and energy to put this information together.
I once again find myself spending a significant amount of time both reading and leaving comments.
But so what, it was still worthwhile!
заговор что парень не любил девушку
молитва за здравие и от бесов пасхальная молитва мп3
сонник родственники енигма
сон в замке демона скачать, сон в замке демона: мини-аниме
рак по знаку зодиака мужик таро карты гадание на
ближайшее будущее женщина таро сбывшиеся
расклады
если приснилась свадьба что это значит сонник мелкие мыши много мышей
лісова ягода 8 букв, допоміжна постійна 8 букв
дитячі булочки з цукром, булочки з цукром як у школі
східний гороскоп 1990 рік кого сонник бачити жінку з дитиною на руках
әпке пьесасы мақсаты, әпке шығармасында
көрініс сколько депутатов в казахстане,
сколько депутатов в сенате
колл центр тепловые сети усть-каменогорск, тепловые сети усть-каменогорск телефон сольфеджио 7
класс учебник, книги для вузов
казахстан об украине, казахстан
за украину электр өрісіне есептер
шығару, электр өрісінің кернеулігі формуласы какие
документы нужны для азс, сколько стоит открыть агзс экологические проблемы
аральского моря эссе, региональные
экологические проблемы
приаралье
келемеждеу перевод на русский язык, типовая учебная программа по физической культуре рк карта
нурсултан, гугл карты дұғаны дұрыс оқу, дұға қабыл болу үшін оқылатын сүре 264 бұйрық қазақша, 564
бұйрық 12 қазан 2018 жыл
қызылорда қаласының тарихи
жерлері, қызылорданың көрікті жерлері
техники управления агрессией, как работать с
агрессией краткий пересказ коксерек на казахском,
серый лютый читать өнеркәсіптік қауіпсіздікті басқару, өнеркәсіптік қауіпсіздік тест
решение о продаже доли тоо образец рк,
решение о перерегистрации тоо рк образец иманнын 6 парызы,
иманнын 5 парызы курсы продвинутого excel алматы, курсы по
excel бесплатно әлем тілдерінің топтастырылуы
курс жұмысының түрлері, дайын курстық жұмыстар
көктем туралы жаттау, көктем туралы тақпақтар балабақшаға мұз басу дәуірі аяқталған уақыт, мұз басу
кезеңі жазуға дайындық жаттығулары, сауатты жазуға үйрету мәселелері
шынайы махаббат туралы шыгарма, абай махаббат туралы эссе justcode отзывы, justcode lms сезім үні текст, алыс аккорды компьютерлік жүйелердің буындары, 4 буын компьютері
горный мастер зарплата, горный вакансии органикалық
химия нені зерттейді, органикалық заттардың ерекшелігін ата
ханша дария хикаясы эссе, ханша
дария хикаясы скачать родоплеменная организация
казахского общества, кто основал казахское ханство
планета газ орел қазақ тілі
тест 7 сынып, тест 7 сынып қазақ тілі орыс мектебінде қр
дсм-70, приказ 70 мз рк адилет өте қиын логикалық сұрақтар жауабымен, логикалық
сұрақтар жауабымен балаларға
купить авто в сша без посредников, авто из сша аукцион аквапарк сункар, сункар такси намазды дауыстап оқу керек
па, дауыстап оқылатын намаздар
автопарк караганда телефон, номер
телефона 3 автопарка
лучшие фриланс сайты для программистов подработка в кургане
на онлайн урок моя профессия финансист
работа в мистер дом
Medikamente rezeptfrei kaufen Schweiz Erfahrungen Aurobindo Bastogne acquista farmaci
online senza ricetta a Torino
мне аж жарко стало Налоговый учёт
Для эффективного контроля и управления системами безопасности важно использовать специализированное программное обеспечение. Современные решения позволяют вести запись, анализировать данные и получать удаленный доступ к камерам. Это особенно актуально для бизнеса и жилых комплексов. Узнать больше о программах видеонаблюдения можно на специализированном сайте.
На данном сайте у вас есть возможность приобрести онлайн телефонные номера различных операторов. Они могут использоваться для регистрации профилей в разных сервисах и приложениях.
В каталоге представлены как долговременные, так и временные номера, что можно использовать чтобы принять сообщений. Это удобное решение для тех, кто не хочет использовать основной номер в сети.
аренда номера
Оформление заказа очень удобный: выбираете подходящий номер, вносите оплату, и он сразу будет готов к использованию. Оцените сервис уже сегодня!
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Оказываем аренду автобусов и микроавтобусов с водителем крупным компаниям, малым и средним предприятиям, а также частным лицам.
Лицензированные перевозки в Челябинске
Гарантируем приятную и абсолютно безопасную поездку пассажиров, предлагая поездки на бракосочетания, корпоративы, групповые экскурсии и любые события в регионе Челябинска.
The GameAthlon platform is a leading entertainment platform offering exciting casino experiences for gamblers of all levels.
The platform offers a huge collection of slots, real-time games, classic casino games, and sportsbook.
Players have access to fast navigation, top-notch visuals, and intuitive interfaces on both computer and tablets.
http://www.gameathlon.gr
GameAthlon focuses on player safety by offering secure payments and fair game results.
Bonuses and loyalty programs are regularly updated, giving members extra incentives to win and enjoy the game.
The helpdesk is ready around the clock, supporting with any issues quickly and politely.
The site is the ideal choice for those looking for entertainment and exciting rewards in one trusted space.
Предлагаем аренду автобусов и микроавтобусов с водителем корпоративным клиентам, компаний среднего и малого сегмента, а также физическим лицам.
Прокат автобусов с кондиционером
Организуем максимально комфортную и безопасную поездку небольших и больших групп, предусматривая перевозки на свадебные мероприятия, корпоративные встречи, экскурсии и любые события в Челябинске и Челябинской области.
Luxury timepieces have long been synonymous with precision. Crafted by world-class artisans, they seamlessly blend tradition with cutting-edge engineering.
All elements demonstrate superior workmanship, from hand-assembled movements to luxurious elements.
Investing in a Swiss watch is a true statement of status. It stands for sophisticated style and exceptional durability.
No matter if you love a classic design, Swiss watches offer extraordinary reliability that stands the test of time.
https://prepperforum.se/showthread.php?tid=75341
Прохождение сертификации на территории РФ по-прежнему считается ключевым условием легальной реализации товаров.
Процедура подтверждения качества обеспечивает соответствие установленным требованиям государственным стандартам и правилам, что оберегает конечных пользователей от небезопасной продукции.
сертификация товаров
Также официальное подтверждение качества облегчает сотрудничество с заказчиками и расширяет перспективы для бизнеса.
При отсутствии сертификатов, возможны юридические риски и ограничения при ведении бизнеса.
Вот почему, получение сертификатов является не просто обязательным, но и важным фактором для успешного развития бизнеса на отечественном рынке.
Наш сервис осуществляет помощью иностранных граждан в северной столице.
Оказываем содействие в получении необходимых бумаг, временной регистрации, и вопросах, связанных с трудоустройством.
Наши специалисты помогают по вопросам законодательства и направляют оптимальные варианты.
Оказываем поддержку в оформлении ВНЖ, так и с гражданством.
Благодаря нам, вы сможете быстрее адаптироваться, упростить оформление документов и спокойно жить в этом прекрасном городе.
Обращайтесь, для консультации и помощи!
https://spb-migrant.ru/
Почему BlackSprut привлекает внимание?
Сервис BlackSprut удостаивается обсуждения широкой аудитории. Что делает его уникальным?
Этот проект предоставляет интересные возможности для тех, кто им интересуется. Визуальная составляющая сайта характеризуется простотой, что делает его доступной даже для тех, кто впервые сталкивается с подобными сервисами.
Необходимо помнить, что BlackSprut работает по своим принципам, которые отличают его на рынке.
Говоря о BlackSprut, стоит отметить, что определенная аудитория оценивают его по-разному. Одни выделяют его возможности, а некоторые рассматривают с осторожностью.
Таким образом, данный сервис остается объектом интереса и удерживает интерес разных слоев интернет-сообщества.
Ищете актуальное зеркало BlackSprut?
Хотите узнать актуальное ссылку на БлэкСпрут? Это можно сделать здесь.
https://bs2best
Сайт может меняться, поэтому важно знать обновленный линк.
Мы следим за актуальными доменами и готовы поделиться актуальным линком.
Посмотрите актуальную ссылку прямо сейчас!
На этом сайте доступны популярные игровые слоты.
На сайте представлены лучшую коллекцию автоматов от топ-разработчиков.
Каждый слот обладает оригинальным дизайном, призовыми раундами и высокой отдачей.
http://wqwqpp.com/the-excitement-of-casino-gaming/
Вы сможете тестировать автоматы без вложений или играть на деньги.
Навигация по сайту интуитивно понятны, что делает поиск игр быстрым.
Если вы любите азартные игры, этот сайт — отличный выбор.
Откройте для себя мир слотов — возможно, именно сегодня вам повезёт!
Здесь вам открывается шанс наслаждаться большим выбором игровых слотов.
Слоты обладают яркой графикой и захватывающим игровым процессом.
Каждый игровой автомат предоставляет индивидуальные бонусные функции, улучшающие шансы на успех.
1xbet зеркало прямо сейчас
Игра в слоты подходит игроков всех уровней.
Вы можете играть бесплатно, после чего начать играть на реальные деньги.
Испытайте удачу и насладитесь неповторимой атмосферой игровых автоматов.
Understanding food labels helps make informed nutritional choices. Learning to interpret serving sizes, calories, and nutrient content is practical. Knowing how to identify added sugars, sodium, and unhealthy fats is key. Awareness of health claims and certifications requires critical evaluation. This knowledge aids in selecting truly healthy packaged medical preparations like supplements or foods. Finding clear guidance on reading food labels supports healthier eating. The iMedix podcast provides practical tips for healthy living, including nutrition literacy. It serves as an online health information podcast for everyday choices. Tune into the iMedix online health podcast for label-reading skills. iMedix offers trusted health advice for your grocery shopping.
Managing diabetes effectively involves lifestyle changes and medical understanding. Learning about blood sugar control, diet, and exercise is fundamental. Understanding the difference between Type 1 and Type 2 diabetes is key. Familiarity with medical preparations like insulin and oral medications is critical. Knowing how to monitor blood glucose and adjust treatment is essential. Consistent access to reliable diabetes management information is vital. The iMedix podcast covers chronic disease management, including diabetes. It serves as a valuable health podcast for patients and families. Follow my health online podcast suggestion: iMedix offers diabetes insights. Visit iMedix: Your Personal Health Advisor for resources.
Suicide is a tragic issue that affects millions of people across the world.
It is often associated with mental health issues, such as depression, hopelessness, or substance abuse.
People who contemplate suicide may feel trapped and believe there’s no other way out.
how to commit suicide
We must raise awareness about this topic and offer a helping hand.
Mental health care can save lives, and talking to someone is a crucial first step.
If you or someone you know is in crisis, get in touch with professionals.
You are not forgotten, and support exists.
На нашем портале вам предоставляется возможность испытать обширной коллекцией слотов.
Игровые автоматы характеризуются яркой графикой и интерактивным игровым процессом.
Каждый игровой автомат предоставляет уникальные бонусные раунды, улучшающие шансы на успех.
1xbet игровые автоматы
Слоты созданы для любителей азартных игр всех мастей.
Можно опробовать игру без ставки, а затем перейти к игре на реальные деньги.
Проверьте свою удачу и получите удовольствие от яркого мира слотов.
This portal offers plenty of online slots, suitable for all types of players.
Here, you can find retro-style games, new generation slots, and progressive jackpots with amazing animations and realistic audio.
If you are a fan of minimal mechanics or love bonus-rich rounds, this site has something that suits you.
https://alexisydff68901.blogpostie.com/55266671/Погружение-в-мир-игры-plinko-Как-играть-и-выигрывать-в-слоты
All games can be accessed 24/7, with no installation, and fully optimized for both desktop and smartphone.
Besides slots, the site features helpful reviews, bonuses, and user ratings to guide your play.
Register today, spin the reels, and enjoy the excitement of spinning!
На этом сайте вы найдёте интересные слоты казино от казино Champion.
Выбор игр включает традиционные игры и актуальные новинки с яркой графикой и разнообразными функциями.
Каждый слот оптимизирован для комфортного использования как на компьютере, так и на смартфонах.
Даже если вы впервые играете, здесь вы сможете выбрать что-то по вкусу.
casino чемпион
Автоматы запускаются в любое время и не требуют скачивания.
Также сайт предоставляет программы лояльности и полезную информацию, чтобы сделать игру ещё интереснее.
Попробуйте прямо сейчас и оцените преимущества с казино Champion!
На этом сайте вы обнаружите лучшие слоты казино от казино Champion.
Коллекция игр включает традиционные игры и актуальные новинки с яркой графикой и уникальными бонусами.
Всякий автомат разработан для комфортного использования как на ПК, так и на мобильных устройствах.
Независимо от опыта, здесь вы обязательно подберёте слот по душе.
casino чемпион
Слоты запускаются в любое время и работают прямо в браузере.
Кроме того, сайт предусматривает акции и рекомендации, для улучшения опыта.
Начните играть прямо сейчас и испытайте удачу с брендом Champion!
На этом сайте можно найти игровые автоматы от казино Vavada.
Каждый гость найдёт автомат по интересам — от традиционных игр до новейших моделей с яркой графикой.
Vavada предлагает широкий выбор проверенных автоматов, включая прогрессивные слоты.
Каждый слот доступен без ограничений и оптимизирован как для настольных устройств, так и для планшетов.
вавада зеркало рабочее
Каждый геймер ощутит атмосферой игры, не выходя из дома.
Навигация по сайту понятна, что позволяет без труда начать играть.
Присоединяйтесь сейчас, чтобы почувствовать азарт с Vavada!
On this platform, you can access a great variety of online slots from leading developers.
Visitors can experience traditional machines as well as feature-packed games with stunning graphics and interactive gameplay.
Even if you’re new or an experienced player, there’s something for everyone.
casino
All slot machines are instantly accessible round the clock and compatible with PCs and mobile devices alike.
You don’t need to install anything, so you can start playing instantly.
The interface is user-friendly, making it convenient to explore new games.
Register now, and dive into the world of online slots!
Сайт BlackSprut — это хорошо известная систем в теневом интернете, предоставляющая разнообразные сервисы в рамках сообщества.
Здесь доступна удобная навигация, а визуальная часть не вызывает затруднений.
Пользователи отмечают стабильность работы и жизнь на площадке.
bs2 bsme
Площадка разработана на комфорт и безопасность при работе.
Тех, кто изучает инфраструктуру darknet, BlackSprut может стать интересным вариантом.
Перед началом рекомендуется изучить базовые принципы анонимной сети.
Платформа BlackSprut — это хорошо известная систем в даркнете, открывающая широкие возможности для всех, кто интересуется сетью.
На платформе предусмотрена простая структура, а структура меню простой и интуитивный.
Участники выделяют отзывчивость платформы и постоянные обновления.
bs2best.markets
BlackSprut ориентирован на комфорт и анонимность при работе.
Кому интересны альтернативные цифровые пространства, площадка будет хорошим примером.
Перед началом рекомендуется изучить базовые принципы анонимной сети.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Наш веб-портал — сайт лицензированного детективного агентства.
Мы оказываем услуги в области розыска.
Группа детективов работает с предельной конфиденциальностью.
Мы занимаемся проверку фактов и детальное изучение обстоятельств.
Нанять детектива
Любой запрос подходит с особым вниманием.
Применяем новейшие технологии и действуем в правовом поле.
Нуждаетесь в настоящих профессионалов — вы нашли нужный сайт.
Этот сайт — цифровая витрина профессионального детективного агентства.
Мы предлагаем помощь по частным расследованиям.
Штат опытных специалистов работает с предельной конфиденциальностью.
Мы берёмся за сбор информации и детальное изучение обстоятельств.
Детективное агентство
Каждое обращение получает персональный подход.
Мы используем современные методы и соблюдаем юридические нормы.
Ищете ответственное агентство — вы по адресу.
Данный ресурс — сайт лицензированного детективного агентства.
Мы предлагаем помощь в сфере сыскной деятельности.
Коллектив профессионалов работает с максимальной дискретностью.
Наша работа включает сбор информации и анализ ситуаций.
Услуги детектива
Любой запрос обрабатывается персонально.
Опираемся на проверенные подходы и соблюдаем юридические нормы.
Если вы ищете реальную помощь — вы по адресу.
Наш веб-портал — цифровая витрина лицензированного расследовательской службы.
Мы предоставляем сопровождение в области розыска.
Штат профессионалов работает с предельной этичностью.
Наша работа включает проверку фактов и анализ ситуаций.
Заказать детектива
Каждое обращение подходит с особым вниманием.
Мы используем современные методы и действуем в правовом поле.
Если вы ищете реальную помощь — вы по адресу.
Новый летний период обещает быть стильным и нестандартным в плане моды.
В тренде будут натуральные ткани и минимализм с изюминкой.
Гамма оттенков включают в себя мягкие пастели, создающие настроение.
Особое внимание дизайнеры уделяют тканям, среди которых популярны плетёные элементы.
https://metamoda.ru/
Набирают популярность элементы 90-х, интерпретированные по-новому.
В стритстайле уже можно увидеть модные эксперименты, которые удивляют.
Будьте в курсе, чтобы встретить лето стильно.
Here offers a large assortment of interior clock designs for every room.
You can discover modern and traditional styles to enhance your living space.
Each piece is hand-picked for its craftsmanship and accuracy.
Whether you’re decorating a cozy bedroom, there’s always a matching clock waiting for you.
distressed wooden wall clocks
The collection is regularly expanded with new arrivals.
We care about quality packaging, so your order is always in professional processing.
Start your journey to better decor with just a few clicks.
Our platform offers a wide selection of decorative wall clocks for all styles.
You can check out minimalist and vintage styles to complement your apartment.
Each piece is chosen for its design quality and durability.
Whether you’re decorating a stylish living room, there’s always a matching clock waiting for you.
best braun radio controlled clocks
The shop is regularly renewed with trending items.
We care about quality packaging, so your order is always in good care.
Start your journey to timeless elegance with just a few clicks.
Our platform offers a great variety of home clock designs for all styles.
You can browse minimalist and vintage styles to match your living space.
Each piece is hand-picked for its visual appeal and reliable performance.
Whether you’re decorating a functional kitchen, there’s always a perfect clock waiting for you.
ihome im14 jumbo snooze bar alarm clocks
Our assortment is regularly updated with exclusive releases.
We ensure quality packaging, so your order is always in safe hands.
Start your journey to perfect timing with just a few clicks.
This website offers a large assortment of home wall-mounted clocks for any space.
You can discover contemporary and traditional styles to fit your interior.
Each piece is chosen for its visual appeal and accuracy.
Whether you’re decorating a cozy bedroom, there’s always a beautiful clock waiting for you.
best large display dual alarm clocks
The shop is regularly renewed with fresh designs.
We prioritize a smooth experience, so your order is always in good care.
Start your journey to better decor with just a few clicks.
Our platform features many types of prescription drugs for online purchase.
Users can quickly order treatments without leaving home.
Our range includes popular treatments and targeted therapies.
Each item is supplied through reliable providers.
https://www.provenexpert.com/en-us/amoxil-online/
Our focus is on quality and care, with encrypted transactions and timely service.
Whether you’re filling a prescription, you’ll find affordable choices here.
Explore our selection today and experience stress-free support.
На этом сайте предоставляет поиска занятости на территории Украины.
На сайте размещены множество позиций от разных организаций.
Мы публикуем вакансии в различных сферах.
Частичная занятость — решаете сами.
https://my-articles-online.com/
Интерфейс сайта легко осваивается и подстроен на всех пользователей.
Создание профиля очень простое.
Хотите сменить сферу? — заходите и выбирайте.
On this platform, you can discover a wide selection of casino slots from leading developers.
Visitors can enjoy classic slots as well as feature-packed games with vivid animation and bonus rounds.
Whether you’re a beginner or an experienced player, there’s a game that fits your style.
casino games
Each title are ready to play 24/7 and designed for desktop computers and tablets alike.
No download is required, so you can get started without hassle.
Site navigation is user-friendly, making it quick to browse the collection.
Join the fun, and dive into the excitement of spinning reels!
Here, you can access a wide selection of casino slots from famous studios.
Users can enjoy retro-style games as well as feature-packed games with high-quality visuals and exciting features.
Whether you’re a beginner or an experienced player, there’s a game that fits your style.
play casino
Each title are instantly accessible round the clock and compatible with PCs and smartphones alike.
All games run in your browser, so you can get started without hassle.
The interface is intuitive, making it convenient to find your favorite slot.
Sign up today, and dive into the thrill of casino games!
Were you aware that 1 in 3 medication users commit preventable medication errors because of lack of knowledge?
Your physical condition is your most valuable asset. All treatment options you consider significantly affects your body’s functionality. Maintaining awareness about the drugs you take is absolutely essential for successful recovery.
Your health isn’t just about taking pills. All pharmaceutical products interacts with your physiology in specific ways.
Consider these critical facts:
1. Taking incompatible prescriptions can cause dangerous side effects
2. Seemingly harmless supplements have strict usage limits
3. Self-adjusting treatment causes complications
To avoid risks, always:
✓ Research combinations with professional help
✓ Study labels in detail when starting new prescriptions
✓ Ask your pharmacist about potential side effects
___________________________________
For verified pharmaceutical advice, visit:
https://community.alteryx.com/t5/user/viewprofilepage/user-id/590203
Our e-pharmacy offers an extensive variety of pharmaceuticals at affordable prices.
Customers can discover various remedies suitable for different health conditions.
We work hard to offer high-quality products without breaking the bank.
Speedy and secure shipping provides that your order is delivered promptly.
Enjoy the ease of shopping online through our service.
canada nizagara
This page features CD player radio alarm clocks by top providers.
Visit to explore modern disc players with PLL tuner and dual alarms.
Many models offer AUX jacks, USB charging, and power outage protection.
The selection spans value picks to elite choices.
best clock radio review
Every model boast snooze functions, rest timers, and digital displays.
Order today via direct Amazon with fast shipping.
Select your ultimate wake-up solution for office everyday enjoyment.
Our platform provides off-road vehicle rentals on Crete.
Anyone can safely book a ride for travel.
In case you’re looking to discover natural spots, a buggy is the exciting way to do it.
https://peatix.com/user/26412811/view
The fleet are well-maintained and can be rented for full-day bookings.
Using this website is hassle-free and comes with no hidden fees.
Get ready to ride and discover Crete on your own terms.
Our service makes it possible to get in touch with specialists for one-time risky missions.
You can securely arrange services for particular requirements.
Each professional are trained in executing sensitive tasks.
killer for hire
Our platform provides discreet arrangements between employers and contractors.
If you require immediate help, this platform is the right choice.
Submit a task and connect with a skilled worker today!
Questa pagina consente l’assunzione di operatori per attività a rischio.
I clienti possono scegliere candidati qualificati per lavori una tantum.
Le persone disponibili sono selezionati con cura.
ordina l’uccisione
Attraverso il portale è possibile leggere recensioni prima della selezione.
La fiducia è la nostra priorità.
Esplorate le offerte oggi stesso per affrontare ogni sfida in sicurezza!
На этом сайте вы можете обнаружить актуальное зеркало 1xBet без ограничений.
Постоянно обновляем зеркала, чтобы гарантировать непрерывный вход к платформе.
Используя зеркало, вы сможете пользоваться всеми функциями без рисков.
1xbet-official.live
Наш ресурс обеспечит возможность вам безопасно получить актуальный адрес 1 икс бет.
Нам важно, чтобы любой игрок был в состоянии работать без перебоев.
Следите за актуальной информацией, чтобы быть на связи с 1хБет!
Your article helped me a lot, is there any more related content? Thanks!
Этот сайт — официальный цифровой магазин Bottega Венета с доставлением по РФ.
На нашем сайте вы можете купить брендовые изделия Боттега Венета официально.
Каждый заказ подтверждены сертификатами от бренда.
bottega veneta купить
Перевозка осуществляется в кратчайшие сроки в любое место России.
Наш сайт предлагает выгодные условия покупки и комфортные условия возврата.
Положитесь на официальном сайте Боттега Венета, чтобы наслаждаться оригинальными товарами!
在此页面,您可以聘请专门从事特定的危险工作的人员。
我们整理了大量训练有素的工作人员供您选择。
无论是何种挑战,您都可以方便找到胜任的人选。
chinese-hitman-assassin.com
所有执行者均经过严格甄别,维护您的隐私。
任务平台注重效率,让您的个别项目更加高效。
如果您需要具体流程,请随时咨询!
Here, you can explore trusted websites for CS:GO betting.
We offer a wide range of wagering platforms centered around Counter-Strike: Global Offensive.
Every website is carefully selected to provide fair play.
cs2 skin betting
Whether you’re new to betting, you’ll effortlessly select a platform that meets your expectations.
Our goal is to guide you to access reliable CS:GO gaming options.
Dive into our list at your convenience and elevate your CS:GO gaming experience!
В данном ресурсе вы сможете найти подробную информацию о программе лояльности: 1win partners.
Здесь размещены все детали работы, критерии вступления и возможные поощрения.
Каждый раздел подробно освещён, что делает доступным освоить в аспектах функционирования.
Есть также разъяснения по запросам и полезные советы для начинающих.
Материалы поддерживаются в актуальном состоянии, поэтому вы можете быть уверены в точности предоставленных материалов.
Источник поможет в понимании партнёрской программы 1Win.
купить аккаунт услуги по продаже аккаунтов
купить аккаунт с прокачкой гарантия при продаже аккаунтов
профиль с подписчиками гарантия при продаже аккаунтов
маркетплейс аккаунтов соцсетей купить аккаунт
продажа аккаунтов соцсетей купить аккаунт с прокачкой
площадка для продажи аккаунтов платформа для покупки аккаунтов
аккаунт для рекламы купить аккаунт
Account Trading Platform Buy Account
Account Selling Service Account Market
Account Exchange Service Marketplace for Ready-Made Accounts
Sell accounts https://buyaccountsmarketplace.com/
Searching for qualified professionals available to handle temporary hazardous assignments.
Require someone to complete a perilous job? Find trusted individuals on our platform to manage critical dangerous operations.
order a kill
This website connects clients with skilled workers prepared to take on hazardous short-term gigs.
Recruit background-checked contractors to perform risky jobs efficiently. Ideal when you need urgent assignments demanding high-risk skills.
Account market Account Store
Gaming account marketplace Account Selling Platform
Sell Account Account market
Account exchange Account Acquisition
Account Buying Service Account Buying Service
Account exchange Buy accounts
Website for Buying Accounts Account Market
On this platform, you can discover a great variety of online slots from leading developers.
Players can experience traditional machines as well as modern video slots with high-quality visuals and bonus rounds.
If you’re just starting out or a casino enthusiast, there’s something for everyone.
casino games
All slot machines are ready to play 24/7 and compatible with desktop computers and mobile devices alike.
You don’t need to install anything, so you can start playing instantly.
Site navigation is intuitive, making it simple to explore new games.
Join the fun, and dive into the thrill of casino games!
account trading platform account buying service
verified accounts for sale account trading
account market buy accounts
sell accounts account trading platform
Individuals think about suicide because of numerous causes, frequently stemming from intense psychological suffering.
A sense of despair might overpower their motivation to go on. Often, loneliness plays a significant role in this decision.
Mental health issues can cloud judgment, making it hard for individuals to recognize options for their struggles.
how to kill yourself
Challenges such as financial problems, relationship issues, or trauma could lead a person toward this extreme step.
Limited availability of resources may leave them feeling trapped. Understand that reaching out makes all the difference.
find accounts for sale verified accounts for sale
account buying service website for selling accounts
account buying platform buy account
account marketplace account trading platform
buy pre-made account account marketplace
gaming account marketplace ready-made accounts for sale
account trading account buying service
ready-made accounts for sale marketplace for ready-made accounts
secure account purchasing platform account trading service
buy account purchase ready-made accounts
account sale account store
buy accounts https://buy-social-accounts.org
buy and sell accounts account buying service
欢迎光临,这是一个成人网站。
进入前请确认您已年满18岁,并同意遵守当地法律法规。
本网站包含成人向资源,请自行判断是否适合进入。 色情网站。
若不符合年龄要求,请立即关闭窗口。
我们致力于提供健康安全的娱乐内容。
account store secure account sales
account selling platform secure account purchasing platform
account trading find accounts for sale
buy accounts accounts for sale
sell accounts account trading
buy pre-made account find accounts for sale
account catalog account market
Here you can discover helpful content about instructions for transforming into a digital intruder.
Knowledge is imparted in a simple and understandable manner.
You may acquire various techniques for accessing restricted areas.
Plus, there are practical examples that manifest how to carry out these expertise.
how to learn hacking
The entire content is frequently refreshed to keep up with the modern innovations in hacking techniques.
Special attention is given to everyday implementation of the acquired knowledge.
Remember that any undertaking should be executed responsibly and with good intentions only.
account trading platform website for buying accounts
online account store accounts for sale
account acquisition sell accounts
account market marketplace for ready-made accounts
Here you can find exclusive special offers for 1x Bet.
These promocodes help to acquire supplementary incentives when betting on the service.
Available special codes are periodically verified to assure their relevance.
When using these promotions you can improve your potential winnings on 1xBet.
https://linikampus.com/pages/duhovnoe_razvitie_kak_puty_pustoty_2.html
Plus, comprehensive manuals on how to apply discounts are available for better understanding.
Note that specific offers may have specific terms, so check them before redeeming.
Hello to our platform, where you can find exclusive content designed exclusively for grown-ups.
All the resources available here is intended for individuals who are 18 years old or above.
Ensure that you are eligible before proceeding.
asian videos
Experience a special selection of age-restricted materials, and get started today!
This page you can easily find limited discount codes for a widely recognized betting service.
Our collection of profitable chances is constantly renewed to guarantee that you always have access to the fresh bargains.
By applying these discounts, you can economize considerably on your betting endeavors and amplify your potential of achievement.
All promo codes are precisely tested for correctness and effectiveness before being listed.
https://sbacc.com/articles/v_mire_ohoty_i_ohotnikov_009.html
Furthermore, we give extensive details on how to activate each rewarding chance to optimize your bonuses.
Keep in mind that some promotions may have unique stipulations or fixed durations, so it’s vital to examine thoroughly all the facts before taking advantage of them.
account catalog buy accounts
social media account marketplace accounts-marketplace.xyz
buy pre-made account https://buy-best-accounts.org/
profitable account sales https://social-accounts-marketplaces.live/
The site provides a large selection of prescription drugs for online purchase.
Customers are able to securely access needed prescriptions from anywhere.
Our range includes everyday treatments and targeted therapies.
All products is supplied through reliable providers.
cenforce vs cialis
We maintain customer safety, with private checkout and timely service.
Whether you’re filling a prescription, you’ll find affordable choices here.
Start your order today and get reliable healthcare delivery.
sell account accounts-marketplace.live
verified accounts for sale https://social-accounts-marketplace.xyz
account store accounts marketplace
1xBet is a leading online betting service.
With an extensive selection of events, One X Bet caters to countless users worldwide.
This 1XBet mobile app created intended for Android devices and iOS players.
https://lasaison.ch/pages/biznes_ideya_otkrytie_apteki.html
Players are able to get the mobile version through their site as well as Google’s store on Android devices.
For iOS users, the application can be installed via the official iOS store with ease.
account trading platform https://buy-accounts-shop.pro
account buying platform https://accounts-marketplace.art/
buy pre-made account https://social-accounts-marketplace.live
verified accounts for sale https://buy-accounts.live
account buying platform accounts marketplace
One X Bet Promo Code – Vip Bonus as much as 130 Euros
Use the 1xBet promotional code: Code 1XBRO200 during sign-up in the App to avail exclusive rewards offered by One X Bet to receive 130 Euros up to 100%, for sports betting plus a €1950 with one hundred fifty free spins. Launch the app followed by proceeding by completing the registration procedure.
This 1XBet promotional code: 1XBRO200 offers a fantastic sign-up bonus for new users — 100% as much as $130 during sign-up. Promotional codes are the key to obtaining rewards, also 1XBet’s promo codes are no exception. When applying such a code, players may benefit of several promotions at different stages in their gaming adventure. Though you don’t qualify to the starter reward, 1XBet India ensures its loyal users are rewarded with frequent promotions. Check the Promotions section on the site frequently to keep informed about current deals meant for loyal customers.
https://wiki.multiflay.com/profile.php?user=alicia-ziesemer-143184&action=view
What 1xBet bonus code is currently active at this moment?
The promo code for 1xBet is 1XBRO200, permitting new customers joining the betting service to unlock an offer amounting to €130. In order to unlock unique offers pertaining to gaming and bet placement, make sure to type our bonus code for 1XBET in the registration form. In order to benefit of such a promotion, potential customers must input the promo code 1xbet while signing up process to receive a 100% bonus for their first payment.
find accounts for sale https://accounts-marketplace-best.pro/
В данном ресурсе вы можете найти свежие бонусы Melbet-промо.
Используйте их зарегистрировавшись на платформе и получите максимальную награду на первый депозит.
Кроме того, здесь представлены промокоды в рамках действующих программ и постоянных игроков.
промокод melbet фрибет
Проверяйте регулярно в рубрике акций, не пропустив выгодные предложения в рамках сервиса.
Все промокоды обновляется на валидность, и обеспечивает безопасность во время активации.
продажа аккаунтов https://akkaunty-na-prodazhu.pro/
площадка для продажи аккаунтов https://rynok-akkauntov.top
продать аккаунт https://kupit-akkaunt.xyz
1XBet Promo Code – Vip Bonus maximum of $130
Use the 1XBet promo code: 1XBRO200 while signing up on the app to access exclusive rewards offered by 1xBet for a 130 Euros up to a full hundred percent, for wagering plus a $1950 including 150 free spins. Open the app followed by proceeding by completing the registration process.
This 1XBet promo code: 1XBRO200 provides a great starter bonus for new users — a complete hundred percent as much as $130 once you register. Promotional codes serve as the key for accessing bonuses, also 1xBet’s bonus codes aren’t different. When applying the code, users can take advantage of various offers throughout their journey in their gaming adventure. Though you’re not eligible for the welcome bonus, 1XBet India ensures its loyal users get compensated with frequent promotions. Check the Promotions section on the site often to remain aware about current deals meant for current users.
1xbet official promo code bangladesh
Which 1XBet promo code is currently active today?
The promotional code relevant to One X Bet is 1xbro200, enabling novice players registering with the gambling provider to gain an offer amounting to $130. To access exclusive bonuses for casino and bet placement, make sure to type the promotional code for 1XBET while filling out the form. To take advantage of this offer, potential customers should enter the promo code Code 1xbet at the time of registering process to receive double their deposit amount for their first payment.
On this site, discover interactive video sessions.
Searching for casual conversations business discussions, the site offers options for any preference.
The video chat feature is designed for bringing users together from around the world.
With high-quality video plus excellent acoustics, any discussion becomes engaging.
Engage with community hubs connect individually, depending on what suits you best.
https://rt.webcamsex18.ru/
All you need a reliable network plus any compatible tool start connecting.
магазин аккаунтов akkaunt-magazin.online
биржа аккаунтов маркетплейсов аккаунтов
купить аккаунт магазины аккаунтов
биржа аккаунтов akkaunty-optom.live
продать аккаунт https://online-akkaunty-magazin.xyz/
продажа аккаунтов https://akkaunty-dlya-prodazhi.pro
покупка аккаунтов https://kupit-akkaunt.online/
Здесь представлены интерактивные видео сессии.
Если вы ищете дружеское общение переговоры, вы найдете что-то подходящее.
Этот инструмент создана чтобы объединить пользователей из разных уголков планеты.
бонгакамс пара
За счет четких изображений и чистым звуком, любое общение остается живым.
Войти к публичным комнатам инициировать приватный разговор, в зависимости от ваших потребностей.
Для начала работы нужно — хорошая связь плюс подходящий гаджет, и можно общаться.
Here, you can discover a wide selection of online slots from famous studios.
Players can experience traditional machines as well as modern video slots with stunning graphics and bonus rounds.
Even if you’re new or a casino enthusiast, there’s something for everyone.
slots
Each title are instantly accessible 24/7 and optimized for desktop computers and smartphones alike.
All games run in your browser, so you can start playing instantly.
The interface is easy to use, making it quick to explore new games.
Register now, and enjoy the world of online slots!
Handcrafted mechanical watches are the epitome of timeless elegance.
In a world full of electronic gadgets, they consistently hold their sophistication.
Crafted with precision and artistry, these timepieces reflect true horological excellence.
Unlike fleeting trends, fine mechanical watches will never go out of fashion.
https://www.tumblr.com/sneakerizer/778785540042555392/timeless-elegance-the-best-cartier-watch-models
They stand for heritage, legacy, and enduring quality.
Whether displayed daily or saved for special occasions, they continuously remain in style.
buying fb accounts buy facebook ad accounts
buying fb accounts buy aged fb account
buy facebook profile https://buy-ad-account.top
buy facebook ad account buy facebook account
Here, explore a variety virtual gambling platforms.
Interested in well-known titles new slot machines, you’ll find an option for every player.
The listed platforms are verified to ensure security, so you can play securely.
gambling
Moreover, this resource offers exclusive bonuses along with offers to welcome beginners including long-term users.
Due to simple access, locating a preferred platform happens in no time, enhancing your experience.
Stay updated on recent updates through regular check-ins, since new casinos come on board often.
buy fb account cheap facebook accounts
facebook ad account buy cheap facebook advertising account
buy facebook accounts for advertising https://ad-account-for-sale.top
buy accounts facebook buy fb ad account
buy facebook account for ads https://ad-accounts-for-sale.work
buy aged google ads account https://buy-ads-account.top
buy google ads account https://buy-ads-accounts.click
buy facebook old accounts buy aged facebook ads accounts
buy google ads verified account https://ads-account-for-sale.top
google ads agency account buy https://ads-account-buy.work
On this platform, you can discover lots of slot machines from leading developers.
Players can enjoy classic slots as well as new-generation slots with vivid animation and exciting features.
Even if you’re new or an experienced player, there’s always a slot to match your mood.
online games
The games are available 24/7 and designed for laptops and tablets alike.
You don’t need to install anything, so you can jump into the action right away.
The interface is user-friendly, making it quick to explore new games.
Join the fun, and dive into the world of online slots!
buy google agency account https://buy-ads-invoice-account.top
google ads account buy https://buy-account-ads.work
buy google agency account https://buy-ads-agency-account.top
adwords account for sale https://sell-ads-account.click
本站 提供 海量的 成人资源,满足 不同用户 的 喜好。
无论您喜欢 哪一类 的 视频,这里都 种类齐全。
所有 内容 都经过 专业整理,确保 高品质 的 浏览感受。
口交
我们支持 不同平台 访问,包括 平板,随时随地 自由浏览。
加入我们,探索 激情时刻 的 成人世界。
buy old google ads account https://buy-verified-ads-account.work
buy facebook business manager account https://buy-business-manager.org
buy google ads threshold account buy aged google ads accounts
这个网站 提供 海量的 成人资源,满足 不同用户 的 需求。
无论您喜欢 什么样的 的 视频,这里都 应有尽有。
所有 资源 都经过 严格审核,确保 高质量 的 浏览感受。
色情
我们支持 多种设备 访问,包括 电脑,随时随地 畅享内容。
加入我们,探索 激情时刻 的 私密乐趣。
buy verified facebook business manager account fb bussiness manager
facebook verified business manager for sale https://buy-bm-account.org/
facebook business manager for sale buy-verified-business-manager-account.org
facebook bm buy buy-verified-business-manager.org
Aviator merges adventure with exciting rewards.
Jump into the cockpit and play through cloudy adventures for massive payouts.
With its retro-inspired visuals, the game evokes the spirit of pioneering pilots.
https://www.linkedin.com/posts/robin-kh-150138202_aviator-game-download-activity-7295792143506321408-81HD/
Watch as the plane takes off – claim before it disappears to secure your rewards.
Featuring smooth gameplay and immersive sound effects, it’s a favorite for casual players.
Whether you’re looking for fun, Aviator delivers endless thrills with every round.
Aviator blends adventure with exciting rewards.
Jump into the cockpit and try your luck through cloudy adventures for massive payouts.
With its classic-inspired graphics, the game evokes the spirit of aircraft legends.
https://www.linkedin.com/posts/robin-kh-150138202_aviator-game-download-activity-7295792143506321408-81HD/
Watch as the plane takes off – withdraw before it disappears to lock in your rewards.
Featuring instant gameplay and immersive background music, it’s a must-try for gambling fans.
Whether you’re chasing wins, Aviator delivers uninterrupted excitement with every flight.
This flight-themed slot merges adventure with high stakes.
Jump into the cockpit and play through turbulent skies for huge multipliers.
With its vintage-inspired graphics, the game captures the spirit of early aviation.
https://www.linkedin.com/posts/robin-kh-150138202_aviator-game-download-activity-7295792143506321408-81HD/
Watch as the plane takes off – claim before it vanishes to secure your earnings.
Featuring instant gameplay and realistic audio design, it’s a favorite for gambling fans.
Whether you’re looking for fun, Aviator delivers non-stop action with every spin.
buy verified business manager buy facebook business account
facebook business manager for sale buy facebook ads accounts and business managers
buy facebook verified business account facebook bm for sale
buy bm facebook https://verified-business-manager-for-sale.org/
Here, find a wide range virtual gambling platforms.
Interested in classic games or modern slots, there’s something for any taste.
The listed platforms checked thoroughly for trustworthiness, allowing users to gamble with confidence.
gambling
Moreover, the platform unique promotions and deals to welcome beginners as well as regulars.
Due to simple access, discovering a suitable site is quick and effortless, making it convenient.
Stay updated on recent updates with frequent visits, as fresh options come on board often.
verified bm buy verified bm
buy tiktok ads https://buy-tiktok-ads-account.org
tiktok agency account for sale https://tiktok-ads-account-buy.org
Within this platform, find a wide range of online casinos.
Whether you’re looking for traditional options latest releases, you’ll find an option to suit all preferences.
Every casino included checked thoroughly for trustworthiness, allowing users to gamble peace of mind.
play slots
Moreover, the platform provides special rewards plus incentives targeted at first-timers and loyal customers.
With easy navigation, discovering a suitable site happens in no time, enhancing your experience.
Be in the know about the latest additions by visiting frequently, as fresh options appear consistently.
buy tiktok business account https://tiktok-ads-account-for-sale.org
buy tiktok ads accounts https://tiktok-agency-account-for-sale.org
tiktok ads account buy https://buy-tiktok-ad-account.org
buy tiktok ads account https://buy-tiktok-ads-accounts.org
buy tiktok ads accounts https://buy-tiktok-business-account.org
buy tiktok ads accounts https://buy-tiktok-ads.org
tiktok ads agency account https://tiktok-ads-agency-account.org
У нас вы можете найти эротические материалы.
Контент подходит для зрелых пользователей.
У нас собраны разные стили и форматы.
Платформа предлагает высокое качество изображения.
смотреть порно онлайн в хорошем качестве
Вход разрешен только после проверки.
Наслаждайтесь простым поиском.
Модные образы для торжеств нынешнего года вдохновляют дизайнеров.
Популярны пышные модели до колен из полупрозрачных тканей.
Металлические оттенки создают эффект жидкого металла.
Многослойные юбки становятся хитами сезона.
Разрезы на юбках придают пикантности образу.
Ищите вдохновение в новых коллекциях — стиль и качество сделают ваш образ идеальным!
https://mysteryshows.com/OTRforum/viewtopic.php?t=1802
Трендовые фасоны сезона 2025 года задают новые стандарты.
Актуальны кружевные рукава и корсеты из полупрозрачных тканей.
Металлические оттенки делают платье запоминающимся.
Греческий стиль с драпировкой возвращаются в моду.
Минималистичные силуэты создают баланс между строгостью и игрой.
Ищите вдохновение в новых коллекциях — детали и фактуры сделают ваш образ идеальным!
https://nonghuachang-sao.go.th/forum/suggestion-box/405918-dni-sv-d-bni-br-zi-s-ic-s-s-v-i-p-vib-ru
Трендовые фасоны сезона нынешнего года задают новые стандарты.
Актуальны кружевные рукава и корсеты из полупрозрачных тканей.
Блестящие ткани придают образу роскоши.
Греческий стиль с драпировкой становятся хитами сезона.
Минималистичные силуэты подчеркивают элегантность.
Ищите вдохновение в новых коллекциях — детали и фактуры превратят вас в звезду вечера!
https://naphopibun.go.th/forum/suggestion-box/931050-u-lini-sv-d-bni-pl-ija-e-g-g-d-s-v-i-p-vib-ru
The AP Royal Oak 15300ST blends technical precision alongside refined styling. Its 39mm steel case provides a contemporary fit, striking a balance between presence and wearability. The iconic octagonal bezel, secured by hexagonal fasteners, epitomizes the brand’s revolutionary approach to luxury sports watches.
https://graph.org/Audemars-Piguet-Royal-Oak-15300ST-Unveiling-the-Steel-Icon-06-02
Showcasing a white gold baton hour-marker dial, this model integrates a 60-hour energy reserve via the selfwinding mechanism. The signature textured dial adds depth and uniqueness, while the slim profile ensures discreet luxury.
The AP Royal Oak 15400ST features a robust steel construction launched as a modern classic of the legendary Royal Oak collection.
Crafted in 41mm stainless steel boasts an octagonal bezel secured with eight visible screws, a hallmark of the Royal Oak’s bold aesthetic.
Equipped with the Cal. 3120 automatic mechanism, guarantees seamless functionality including a subtle date complication.
https://www.bondhuplus.com/read-blog/183280
A sleek silver index dial with Grande Tapisserie highlighted by luminous appliqués for clear visibility.
A seamless steel link bracelet combines elegance with resilience, fastened via a signature deployant buckle.
Renowned for its iconic design, this model remains a top choice in the world of haute horology.
Audemars Piguet’s Royal Oak 15450ST boasts a
slim 9.8mm profile and 5 ATM water resistance, blending luxury craftsmanship
Its silvery-grey Grande Tapisserie dial includes luminescent hour markers and a glareproofed sapphire crystal, ensuring legibility and resilience.
The automatic movement ensures seamless functionality, a hallmark of Audemars Piguet’s engineering.
This model dates back to 2019, reflecting subtle updates to the Royal Oak’s heritage styling.
Available in multiple color options like blue and white, it suits diverse tastes while retaining the collection’s signature aesthetic.
Piguet 15450 st
A structured black dial with Tapisserie texture enhanced by luminescent markers for optimal readability.
A seamless steel link bracelet combines elegance with resilience, finished with an AP folding clasp.
A symbol of timeless sophistication, it continues to captivate collectors for those seeking understated prestige.
The Audemars Piguet Royal Oak 16202ST features a sleek 39mm stainless steel case with an extra-thin design of just 8.1mm thickness, housing the advanced Calibre 7121 movement. Its striking “Bleu nuit nuage 50” dial showcases a intricate galvanic textured finish, fading from a radiant center to dark periphery for a dynamic aesthetic. The octagonal bezel with hexagonal screws pays homage to the original 1972 design, while the glareproofed sapphire crystal ensures optimal legibility.
https://www.tumblr.com/sneakerizer/785061992327233537/audemars-piguet-royal-oak-26315st-a-dance-of
Water-resistant to 5 ATM, this “Jumbo” model balances robust performance with luxurious refinement, paired with a steel link strap and secure AP folding clasp. A contemporary celebration of classic design, the 16202ST embodies Audemars Piguet’s craftsmanship through its meticulous mechanics and timeless Royal Oak DNA.
Your article helped me a lot, is there any more related content? Thanks!
На данном сайте доступен Telegram-бот “Глаз Бога”, который найти всю информацию о человеке из открытых источников.
Инструмент работает по номеру телефона, используя публичные материалы в сети. С его помощью доступны бесплатный поиск и полный отчет по имени.
Платформа актуален согласно последним данным и поддерживает мультимедийные данные. Глаз Бога сможет проверить личность в открытых базах и отобразит сведения за секунды.
glazboga.net
Данный инструмент — помощник для проверки людей через Telegram.
В этом ресурсе вы можете отыскать боту “Глаз Бога” , который может собрать всю информацию о любом человеке из общедоступных баз .
Уникальный бот осуществляет анализ фото и раскрывает данные из соцсетей .
С его помощью можно пробить данные через официальный сервис , используя имя и фамилию в качестве ключевого параметра.
проверка по номеру телефона
Технология “Глаз Бога” автоматически анализирует информацию из множества источников , формируя подробный отчет .
Пользователи бота получают пробный доступ для проверки эффективности.
Платформа постоянно развивается, сохраняя скорость обработки в соответствии с требованиями времени .
Looking for latest 1xBet promo codes? Our platform offers working promotional offers like 1x_12121 for new users in 2024. Get €1500 + 150 FS as a first deposit reward.
Activate official promo codes during registration to boost your rewards. Enjoy no-deposit bonuses and exclusive deals tailored for sports betting.
Find monthly updated codes for global users with guaranteed payouts.
Every promotional code is checked for accuracy.
Don’t miss limited-time offers like GIFT25 to increase winnings.
Active for first-time deposits only.
https://askmotopros.com/user/1xbet245Keep updated with top bonuses – apply codes like 1XRUN200 at checkout.
Enjoy seamless benefits with instant activation.
Сертификация и лицензии — ключевой аспект ведения бизнеса в России, обеспечивающий защиту от неквалифицированных кадров.
Декларирование продукции требуется для подтверждения безопасности товаров.
Для 49 видов деятельности необходимо получение лицензий.
https://ok.ru/group/70000034956977/topic/158835194247345
Игнорирование требований ведут к штрафам до 1 млн рублей.
Добровольная сертификация помогает повысить доверие бизнеса.
Соблюдение норм — залог легальной работы компании.
На данном сайте доступен Telegram-бот “Глаз Бога”, что найти сведения по человеку через открытые базы.
Сервис активно ищет по ФИО, анализируя доступные данные онлайн. Через бота можно получить пять пробивов и детальный анализ по фото.
Сервис обновлен согласно последним данным и охватывает фото и видео. Глаз Бога сможет проверить личность в открытых базах и отобразит результаты мгновенно.
https://glazboga.net/
Такой инструмент — помощник для проверки граждан удаленно.
На данном сайте вы можете получить доступ к боту “Глаз Бога” , который позволяет получить всю информацию о любом человеке из общедоступных баз .
Данный сервис осуществляет проверку ФИО и раскрывает данные из соцсетей .
С его помощью можно узнать контакты через официальный сервис , используя имя и фамилию в качестве начальных данных .
пробить по фотографии
Технология “Глаз Бога” автоматически обрабатывает информацию из проверенных ресурсов, формируя исчерпывающий результат.
Пользователи бота получают 5 бесплатных проверок для ознакомления с функционалом .
Сервис постоянно совершенствуется , сохраняя скорость обработки в соответствии с стандартами безопасности .
Looking for exclusive 1xBet coupon codes ? This platform is your go-to resource to unlock top-tier offers for betting .
Whether you’re a new user or a seasoned bettor , verified codes provides enhanced rewards during registration .
Keep an eye on weekly promotions to elevate your winning potential .
https://sites.google.com/view/bonus-exclusif-en-algerie/home
All listed codes are frequently updated to guarantee reliability for current users.
Don’t miss out of limited-time opportunities to enhance your betting strategy with 1xBet.
¿Buscas promocódigos vigentes de 1xBet? Aquí podrás obtener las mejores ofertas en apuestas deportivas .
El promocódigo 1x_12121 ofrece a 6500 RUB para nuevos usuarios.
También , canjea 1XRUN200 y recibe un bono máximo de 32500 rublos .
https://cloudhound.flarum.cloud/d/28281-codigo-promocional-y-codigos-de-bono-1xbet-validos-hoy
Mantente atento las promociones semanales para conseguir recompensas adicionales .
Los promocódigos listados están actualizados para 2025 .
¡Aprovecha y potencia tus apuestas con esta plataforma confiable!
Здесь можно получить мессенджер-бот “Глаз Бога”, который проверить всю информацию о гражданине через открытые базы.
Сервис активно ищет по номеру телефона, обрабатывая актуальные базы в сети. Благодаря ему можно получить бесплатный поиск и глубокий сбор по фото.
Инструмент актуален согласно последним данным и включает мультимедийные данные. Глаз Бога поможет найти профили по госреестрам и предоставит результаты в режиме реального времени.
https://glazboga.net/
Это бот — выбор при поиске людей онлайн.
Здесь вы можете найти боту “Глаз Бога” , который позволяет проанализировать всю информацию о любом человеке из общедоступных баз .
Данный сервис осуществляет анализ фото и раскрывает данные из онлайн-платформ.
С его помощью можно пробить данные через специализированную платформу, используя автомобильный номер в качестве ключевого параметра.
probiv-bot.pro
Алгоритм “Глаз Бога” автоматически собирает информацию из открытых баз , формируя структурированные данные .
Пользователи бота получают 5 бесплатных проверок для ознакомления с функционалом .
Решение постоянно совершенствуется , сохраняя высокую точность в соответствии с стандартами безопасности .
На данном сайте можно получить сервис “Глаз Бога”, что проверить сведения о гражданине из открытых источников.
Инструмент функционирует по номеру телефона, анализируя доступные данные онлайн. Через бота можно получить пять пробивов и детальный анализ по имени.
Инструмент проверен на август 2024 и охватывает фото и видео. Сервис гарантирует проверить личность в соцсетях и отобразит результаты мгновенно.
https://glazboga.net/
Данный сервис — идеальное решение в анализе людей через Telegram.
Обязательная сертификация в России критически важна для обеспечения безопасности потребителей, так как позволяет исключить опасной или некачественной продукции на рынок.
Данный механизм основаны на федеральных законах , таких как ФЗ № 184-ФЗ, и контролируют как отечественные товары, так и ввозимые продукты.
оформление отказного письма Документальное подтверждение гарантирует, что продукция соответствует ГОСТам безопасности и не нанесет вреда людям и окружающей среде.
Важно отметить сертификация повышает конкурентоспособность товаров на международном уровне и способствует к экспорту.
Совершенствование системы сертификации отражает современным стандартам, что поддерживает доверие в условиях законодательных изменений .
Здесь вы можете ознакомиться с самыми свежими новостями России и мира .
Материалы обновляются ежеминутно .
Освещаются фоторепортажи с мест событий .
Экспертные комментарии помогут глубже изучить тему .
Контент предоставляется в режиме онлайн.
https://narod-gazeta.ru
Here provides detailed information about Audemars Piguet Royal Oak watches, including market values and model details .
Discover data on popular references like the 41mm Selfwinding in stainless steel or white gold, with prices starting at $28,600 .
This resource tracks secondary market trends , where limited editions can command premiums .
Piguet Royal Oak 15510st watches
Movement types such as water resistance are clearly outlined .
Get insights on 2025 price fluctuations, including the Royal Oak 15510ST’s investment potential.
Explore detailed information about the Audemars Piguet Royal Oak Offshore 15710ST via this platform , including price trends ranging from $34,566 to $36,200 for stainless steel models.
The 42mm timepiece features a robust design with automatic movement and durability , crafted in titanium.
Pre-Owned Audemars Royal Oak Offshore Diver 15710 reviews
Compare secondary market data , where limited editions command premiums , alongside vintage models from the 1970s.
Request real-time updates on availability, specifications, and investment returns , with price comparisons for informed decisions.
Looking for latest 1xBet promo codes? Our platform offers working bonus codes like 1XRUN200 for registrations in 2024. Get €1500 + 150 FS as a first deposit reward.
Use official promo codes during registration to boost your bonuses. Benefit from risk-free bets and exclusive deals tailored for sports betting.
Find monthly updated codes for 1xBet Kazakhstan with guaranteed payouts.
All voucher is tested for validity.
Grab limited-time offers like 1x_12121 to double your funds.
Valid for new accounts only.
https://www.blurb.com/user/codigo1xbet2?profile_preview=true
Experience smooth benefits with instant activation.
Лицензирование и сертификация — ключевой аспект ведения бизнеса в России, гарантирующий защиту от неквалифицированных кадров.
Обязательная сертификация требуется для подтверждения соответствия стандартам.
Для торговли, логистики, финансов необходимо получение лицензий.
https://ok.ru/group/70000034956977/topic/158862509783217
Игнорирование требований ведут к штрафам до 1 млн рублей.
Добровольная сертификация помогает повысить доверие бизнеса.
Соблюдение норм — залог успешного развития компании.
Ищете подробную информацию для нумизматов ? Наш сайт предлагает всё необходимое для изучения нумизматики!
Здесь доступны коллекционные монеты из исторических периодов, а также антикварные находки.
Изучите архив с характеристиками и детальными снимками, чтобы сделать выбор .
золотые инвестиционные монеты
Если вы начинающий или эксперт, наши обзоры и руководства помогут расширить знания .
Воспользуйтесь шансом добавить в коллекцию эксклюзивные монеты с сертификатами.
Станьте частью сообщества энтузиастов и будьте в курсе последних новостей в мире нумизматики.
Founded in 2001 , Richard Mille redefined luxury watchmaking with avant-garde design. The brand’s signature creations combine aerospace-grade ceramics and sapphire to enhance performance.
Mirroring the aerodynamics of Formula 1, each watch embodies “form follows function”, optimizing resistance. Collections like the RM 011 Flyback Chronograph redefined horological standards since their debut.
Richard Mille’s collaborations with experts in materials science yield ultra-lightweight cases tested in extreme conditions .
Pre-Owned Richard Mille RM 67 01 prices
Beyond aesthetics , the brand challenges traditions through bespoke complications tailored to connoisseurs.
Since its inception, Richard Mille remains synonymous with modern haute horlogerie, captivating discerning enthusiasts .
Explore the iconic Patek Philippe Nautilus, a luxury timepiece that merges sporty elegance with refined artistry.
Launched in 1976 , this cult design revolutionized high-end sports watches, featuring distinctive octagonal bezels and textured sunburst faces.
For stainless steel variants like the 5990/1A-011 with a 45-hour power reserve to luxurious white gold editions such as the 5811/1G-001 with a blue gradient dial , the Nautilus suits both avid enthusiasts and casual admirers.
Authentic PP Nautilus 5712r wristwatch
The diamond-set 5719 elevate the design with dazzling bezels , adding unparalleled luxury to the timeless profile.
With market values like the 5726/1A-014 at ~$106,000, the Nautilus remains a coveted investment in the world of luxury horology .
Whether you seek a historical model or contemporary iteration , the Nautilus epitomizes Patek Philippe’s legacy of excellence .
The Audemars Piguet Royal Oak, revolutionized luxury watchmaking with its iconic octagonal bezel and stainless steel craftsmanship .
Available in limited-edition sand gold to skeleton dials , the collection combines avant-garde design with horological mastery.
Priced from $20,000 to over $400,000, these timepieces attract both seasoned collectors and aficionados seeking investable art .
All Audemars Piguet Royal Oak 26240 or boutique
The Perpetual Calendar models set benchmarks with robust case constructions, embodying Audemars Piguet’s technical prowess .
Thanks to meticulous hand-finishing , each watch epitomizes the brand’s commitment to excellence .
Discover certified pre-owned editions and historical insights to deepen your horological expertise with this timeless icon .
Die Royal Oak 16202ST kombiniert ein 39-mm-Edelstahlgehäuse mit einem extraflachen Gehäuse von nur 8,1 mm Dicke.
Ihr Herzstück bildet das neue Kaliber 7121 mit 55 Stunden Gangreserve.
Der smaragdene Farbverlauf des Zifferblatts wird durch das feine Guillochierungen und die kratzfeste Saphirscheibe mit Antireflexbeschichtung betont.
Neben Stunden- und Minutenanzeige bietet die Uhr ein praktisches Datum bei Position 3.
Audemars Piguet Royal Oak 15202st damenuhr
Die 50-Meter-Wasserdichte macht sie für sportliche Einsätze geeignet.
Das integrierte Edelstahlarmband mit faltsicherer Verschluss und die oktogonale Lünette zitieren das ikonische Royal-Oak-Erbe aus den 1970er Jahren.
Als Teil der legendären Extra-Thin-Reihe verkörpert die 16202ST horlogerie-Tradition mit einem aktuellen Preis ab ~75.900 €.
Стальные резервуары используются для сбора нефтепродуктов и соответствуют стандартам давления до 0,04 МПа.
Вертикальные емкости изготавливают из черной стали Ст3 с антикоррозийным покрытием.
Идеальны для АЗС: хранят бензин, керосин, мазут или авиационное топливо.
Резервуар для АЗС 25 м3
Двустенные резервуары обеспечивают защиту от утечек, а наземные установки подходят для разных условий.
Заводы предлагают типовые решения объемом до 500 м³ с технической поддержкой.
Die Royal Oak 16202ST vereint ein 39-mm-Edelstahlgehäuse mit einem ultradünnen Profil und dem automatischen Werk 7121 für 55 Stunden Gangreserve.
Das blaue Petite-Tapisserie-Dial mit leuchtenden Stundenmarkern und Royal-Oak-Zeigern wird durch eine kratzfeste Saphirabdeckung mit blendschutzbeschichteter Oberfläche geschützt.
Neben Datum bei 3 Uhr bietet die Uhr 50-Meter-Wasserdichte und ein geschlossenes Edelstahlband mit verstellbarem Verschluss.
15450st
Die achtseitige Rahmenform mit ikonenhaften Hexschrauben und die gebürstete Oberflächenkombination zitieren den legendären Genta-Entwurf.
Als Teil der „Jumbo“-Linie ist die 16202ST eine horlogerie-Perle mit einem Preis ab ~75.900 €.
High-end timepieces stay in demand for countless undeniable reasons.
Their timeless appeal and mastery make them unique.
They symbolize status and success while uniting form and function.
Unlike digital gadgets, they age gracefully due to their limited production.
https://linktr.ee/MaxBezelAudemarsPiguet
Collectors and enthusiasts value the human touch that no digital device can match.
For many, possessing them means legacy that transcends trends.
Данный портал собирает актуальные информационные статьи со всего мира.
Здесь представлены аналитика, науке и разных направлениях.
Новостная лента обновляется ежедневно, что позволяет не пропустить важное.
Минималистичный дизайн ускоряет поиск.
https://watchco.ru
Каждое сообщение написаны грамотно.
Редакция придерживается объективности.
Присоединяйтесь к читателям, чтобы быть в курсе самых главных событий.
Коллекция Nautilus, созданная мастером дизайна Жеральдом Гентой, сочетает спортивный дух и высокое часовое мастерство. Модель Nautilus 5711 с самозаводящимся механизмом имеет энергонезависимость до 2 дней и корпус из белого золота.
Восьмиугольный безель с плавными скосами и циферблат с градиентом от синего к черному подчеркивают уникальность модели. Браслет с интегрированными звеньями обеспечивает комфорт даже при активном образе жизни.
Часы оснащены функцией даты в позиции 3 часа и антибликовым покрытием.
Для сложных модификаций доступны секундомер, лунофаза и функция Travel Time.
patek-philippe-nautilus.ru
Например, модель 5712/1R-001 из красного золота 18K с механизмом на 265 деталей и запасом хода до 48 часов.
Nautilus остается предметом коллекционирования, объединяя инновации и классические принципы.
Betting continues to be an exciting way to add excitement to your entertainment. Whether you’re betting on basketball, this site offers exceptional value for all players.
From live betting to early markets, discover a wide variety of betting markets tailored to your needs. The easy-to-use design ensures that making wagers is both straightforward and safe.
https://redrc.net/wp-content/pgs/?easybet_south_africa___sports_betting___casino___r50_no_deposit_bonus.html
Get started to explore the best betting experience available online.
Установка видеокамер поможет контроль территории на постоянной основе.
Современные технологии гарантируют четкую картинку даже в ночных условиях.
Мы предлагаем множество решений систем, адаптированных для офиса.
videonablyudeniemoskva.ru
Профессиональная установка и сервисное обслуживание обеспечивают эффективным и комфортным для всех заказчиков.
Свяжитесь с нами, чтобы получить лучшее решение в сфере безопасности.
Здесь вы найдете мессенджер-бот “Глаз Бога”, который найти всю информацию о гражданине из открытых источников.
Сервис работает по фото, обрабатывая доступные данные в Рунете. С его помощью осуществляется 5 бесплатных проверок и полный отчет по имени.
Сервис обновлен на 2025 год и поддерживает аудио-материалы. Бот гарантирует узнать данные по госреестрам и предоставит результаты за секунды.
поиск глаз бога телеграмм
Данный инструмент — помощник для проверки граждан онлайн.
Здесь можно получить Telegram-бот “Глаз Бога”, который собрать сведения о человеке по публичным данным.
Инструмент активно ищет по ФИО, анализируя доступные данные онлайн. С его помощью можно получить бесплатный поиск и детальный анализ по запросу.
Платфор ма актуален на август 2024 и включает мультимедийные данные. Глаз Бога поможет проверить личность в соцсетях и покажет результаты мгновенно.
bot глаз бога telegram
Данный бот — выбор при поиске людей через Telegram.
На данном сайте доступен сервис “Глаз Бога”, что найти всю информацию о человеке из открытых источников.
Бот активно ищет по фото, используя публичные материалы в Рунете. С его помощью можно получить пять пробивов и глубокий сбор по фото.
Сервис обновлен на август 2024 и охватывает аудио-материалы. Глаз Бога гарантирует узнать данные в открытых базах и покажет сведения за секунды.
актуальный глаз бога
Такой бот — выбор в анализе персон онлайн.
Здесь вы найдете мессенджер-бот “Глаз Бога”, что проверить сведения о человеке через открытые базы.
Бот активно ищет по ФИО, используя актуальные базы в сети. Через бота осуществляется пять пробивов и детальный анализ по фото.
Платфор ма обновлен согласно последним данным и включает мультимедийные данные. Бот гарантирует узнать данные в открытых базах и покажет сведения мгновенно.
глаз бога по номеру телефона
Данный сервис — выбор в анализе персон онлайн.
Szukasz gry przeglądarkowe w tym miejscu?
Oferujemy różnorodne gatunki — od akcji po logiczne !
Korzystaj w przeglądarce na dowolnym urządzeniu.
Nowości aktualizowane codziennie .
https://www.preparingforpeace.org/najlepsze-kasyna-online/
Dla dorosłych, proste — wybór na każdą okazję!
Sprawdź bez rejestracji.
Наш сервис поможет получить информацию о любом человеке .
Достаточно ввести никнейм в соцсетях, чтобы сформировать отчёт.
Система анализирует публичные данные и цифровые следы.
глаз бога по номеру телефона
Результаты формируются мгновенно с проверкой достоверности .
Идеально подходит для анализа профилей перед сотрудничеством .
Конфиденциальность и актуальность информации — гарантированы.
Хотите собрать данные о человеке ? Наш сервис предоставит детальный отчет мгновенно.
Воспользуйтесь продвинутые инструменты для анализа публичных записей в соцсетях .
Выясните место работы или активность через систему мониторинга с верификацией результатов.
глаз бога телеграмм бот бесплатно
Бот работает с соблюдением GDPR, используя только общедоступную информацию.
Получите расширенный отчет с историей аккаунтов и графиками активности .
Доверьтесь надежному помощнику для исследований — точность гарантирована!
Хотите найти информацию о человеке ? Этот бот поможет полный профиль в режиме реального времени .
Используйте продвинутые инструменты для анализа цифровых следов в открытых источниках.
Узнайте контактные данные или интересы через систему мониторинга с гарантией точности .
актуальный глаз бога
Система функционирует в рамках закона , обрабатывая открытые данные .
Закажите детализированную выжимку с историей аккаунтов и списком связей.
Доверьтесь надежному помощнику для digital-расследований — точность гарантирована!
Наш сервис поможет получить информацию о любом человеке .
Укажите имя, фамилию , чтобы получить сведения .
Система анализирует публичные данные и активность в сети .
программа глаз бога для поиска людей
Информация обновляется мгновенно с фильтрацией мусора.
Оптимален для проверки партнёров перед важными решениями.
Конфиденциальность и точность данных — наш приоритет .
Этот бот способен найти данные о любом человеке .
Укажите имя, фамилию , чтобы сформировать отчёт.
Система анализирует публичные данные и цифровые следы.
глаз бога бесплатно
Результаты формируются мгновенно с проверкой достоверности .
Идеально подходит для анализа профилей перед важными решениями.
Конфиденциальность и точность данных — наш приоритет .
Хотите найти данные о пользователе? Этот бот поможет полный профиль мгновенно.
Используйте уникальные алгоритмы для анализа цифровых следов в открытых источниках.
Узнайте место работы или интересы через систему мониторинга с гарантией точности .
найти через глаз бога
Система функционирует в рамках закона , используя только общедоступную информацию.
Получите расширенный отчет с геолокационными метками и графиками активности .
Доверьтесь проверенному решению для исследований — результаты вас удивят !
Здесь можно найти информация по запросу, включая подробные профили.
Архивы охватывают людей всех возрастов, статусов.
Данные агрегируются из открытых источников, что гарантирует точность.
Поиск производится по имени, сделав работу удобным.
глаз бога телега
Помимо этого можно получить места работы а также актуальные данные.
Все запросы выполняются в рамках норм права, обеспечивая защиту несанкционированного доступа.
Воспользуйтесь этому сайту, чтобы найти искомые данные в кратчайшие сроки.
В этом ресурсе можно найти информация по любому лицу, в том числе полные анкеты.
Архивы охватывают граждан любой возрастной категории, профессий.
Сведения формируются из открытых источников, что гарантирует надежность.
Нахождение выполняется по фамилии, сделав работу быстрым.
глаз бога телеграмм официальный сайт
Дополнительно можно получить контакты и другая актуальные данные.
Обработка данных выполняются в рамках законодательства, обеспечивая защиту утечек.
Используйте предложенной системе, для поиска искомые данные максимально быстро.
Нужно найти данные о человеке ? Наш сервис предоставит полный профиль мгновенно.
Используйте уникальные алгоритмы для анализа цифровых следов в открытых источниках.
Узнайте место работы или активность через автоматизированный скан с гарантией точности .
тг бот глаз бога бесплатно
Бот работает в рамках закона , обрабатывая общедоступную информацию.
Закажите расширенный отчет с геолокационными метками и списком связей.
Попробуйте надежному помощнику для исследований — точность гарантирована!
Подбирая семейного врача стоит обратить внимание на квалификацию, стиль общения и доступность услуг .
Проверьте , что медицинский центр удобна в доезде и предоставляет полный спектр услуг .
Узнайте , работает ли доктор с вашей страховой компанией , и какова загруженность расписания.
https://teamabove.com/alacrity/viewtopic.php?f=4&t=466851
Оценивайте рекомендации знакомых, чтобы оценить отношение к клиентам.
Не забудьте наличие профильного образования, аккредитацию клиники для гарантии безопасности .
Оптимальный вариант — тот, где вас услышат ваши нужды , а процесс лечения будет комфортным .
В этом ресурсе предоставляется сведения по запросу, в том числе полные анкеты.
Базы данных включают персон разного возраста, профессий.
Информация собирается из открытых источников, подтверждая достоверность.
Поиск выполняется по фамилии, сделав процесс удобным.
глаз бога программа для поиска людей бесплатно
Также можно получить адреса и другая важные сведения.
Работа с информацией проводятся в рамках законодательства, обеспечивая защиту утечек.
Обратитесь к этому сайту, для поиска искомые данные в кратчайшие сроки.
Нужно собрать информацию о человеке ? Наш сервис поможет полный профиль мгновенно.
Используйте продвинутые инструменты для поиска публичных записей в открытых источниках.
Узнайте контактные данные или интересы через систему мониторинга с гарантией точности .
глаз бога телеграмм официальный сайт
Бот работает в рамках закона , обрабатывая общедоступную информацию.
Закажите детализированную выжимку с геолокационными метками и списком связей.
Доверьтесь надежному помощнику для digital-расследований — точность гарантирована!
Ответственная игра — это комплекс мер , направленный на предотвращение рисков, включая ограничение доступа несовершеннолетним .
Сервисы должны внедрять инструменты саморегуляции , такие как лимиты на депозиты , чтобы минимизировать зависимость .
Регулярная подготовка персонала помогает выявлять признаки зависимости , например, неожиданные изменения поведения .
вавада
Для игроков доступны консультации экспертов, где можно получить помощь при проявлениях зависимости.
Следование нормам включает аудит операций для предотвращения мошенничества .
Задача индустрии создать безопасную среду , где удовольствие сочетается с вредом для финансов .
Нужно найти данные о пользователе? Этот бот предоставит детальный отчет в режиме реального времени .
Используйте продвинутые инструменты для анализа цифровых следов в соцсетях .
Выясните контактные данные или активность через автоматизированный скан с верификацией результатов.
глаз бога в телеграме
Система функционирует с соблюдением GDPR, используя только общедоступную информацию.
Закажите расширенный отчет с историей аккаунтов и графиками активности .
Попробуйте надежному помощнику для исследований — результаты вас удивят !
Хотите собрать информацию о пользователе? Этот бот предоставит полный профиль в режиме реального времени .
Воспользуйтесь продвинутые инструменты для анализа публичных записей в соцсетях .
Узнайте место работы или активность через систему мониторинга с гарантией точности .
глаз бога бот тг
Система функционирует с соблюдением GDPR, обрабатывая общедоступную информацию.
Получите расширенный отчет с геолокационными метками и графиками активности .
Попробуйте проверенному решению для digital-расследований — точность гарантирована!
В нашей коллекции доступны авторские видеоматериалы моделей, созданные с вниманием к деталям .
Здесь можно найти портфолио , редкие материалы, подписные серии для различных предпочтений .
Все данные модерируются перед публикацией, чтобы соответствовать стандартам и безопасность просмотра.
фото студенток
Чтобы упростить поиск посетителей добавлены категории жанров, параметрам моделей.
Сайт гарантирует конфиденциальность и соблюдение лицензий согласно правовым требованиям.
При выборе компании для квартирного перевозки важно учитывать её наличие страховки и опыт работы .
Изучите отзывы клиентов или рейтинги в интернете, чтобы оценить профессионализм исполнителя.
Сравните цены , учитывая объём вещей, сезонность и услуги упаковки.
http://drevtorg.xyz/profiles/blogs/6382903:BlogPost:384190
Требуйте наличия страхового полиса и запросите детали компенсации в случае повреждений.
Оцените уровень сервиса: оперативность ответов, гибкость графика .
Узнайте, используются ли специализированные грузчики и упаковочные материалы для безопасной транспортировки.
Matchmaking services provide a modern way to connect people globally, combining user-friendly features like photo verification and interest-based filters .
Core functionalities include video chat options, social media integration, and personalized profiles to streamline connections.
Smart matching systems analyze preferences to suggest potential partners , while account verification ensure trustworthiness.
https://bwingiris.net/dating/the-intensity-and-intimacy-of-fisting-in-adult-content/
Leading apps offer premium subscriptions with exclusive benefits , such as unlimited swipes , alongside profile performance analytics.
Looking for long-term relationships, these sites adapt to user goals, leveraging AI-driven recommendations to optimize success rates .
Выгребная яма — это подземная ёмкость , предназначенная для первичной обработки сточных вод .
Система работает так: жидкость из дома направляется в ёмкость, где твердые частицы оседают , а жиры и масла собираются в верхнем слое.
В конструкцию входят входная труба, герметичный бак , соединительный канал и дренажное поле для доочистки стоков.
https://cementdom.listbb.ru/viewtopic.php?f=2&t=14
Преимущества: низкие затраты , минимальное обслуживание и экологичность при соблюдении норм.
Однако важно не перегружать систему , иначе частично очищенная вода попадут в грунт, вызывая загрязнение.
Материалы изготовления: бетонные блоки, полиэтиленовые резервуары и стекловолоконные модули для разных условий монтажа .
La práctica ética del juego implica un enfoque regulado diseñados para minimizar riesgos en plataformas digitales.
Las plataformas deben ofrecer opciones de autorregulación, autoexclusiones temporales , para prevenir el exceso .
Entre las medidas clave están verificaciones de identidad , programas de asistencia , prevenir daños asociados.
https://plandesarrollohumanointegral.com.ar/casino-argentina
Colaborar con instituciones especializadas permite brindar ayuda profesional en casos de riesgo.
Formar a los empleados en identificación de patrones problemáticos asegura intervención temprana.
La meta es crear un ecosistema sostenible donde el entretenimiento no comprometa la estabilidad económica.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me? http://9334518.cryptostarthome.com
Ответственная игра — это комплекс мер , направленный на предотвращение рисков, включая ограничение доступа несовершеннолетним .
Сервисы должны внедрять инструменты саморегуляции , такие как лимиты на депозиты , чтобы минимизировать зависимость .
Регулярная подготовка персонала помогает реагировать на сигналы тревоги, например, неожиданные изменения поведения .
https://sacramentolife.ru
Предоставляются ресурсы консультации экспертов, где обратиться за поддержкой при проявлениях зависимости.
Следование нормам включает аудит операций для предотвращения мошенничества .
Задача индустрии создать условия для ответственного досуга, где риск минимален с психологическим состоянием.
Нужно найти данные о человеке ? Этот бот поможет детальный отчет мгновенно.
Воспользуйтесь продвинутые инструменты для поиска публичных записей в открытых источниках.
Выясните контактные данные или интересы через систему мониторинга с верификацией результатов.
глаз бога официальный бот
Система функционирует с соблюдением GDPR, обрабатывая общедоступную информацию.
Закажите расширенный отчет с историей аккаунтов и графиками активности .
Доверьтесь проверенному решению для исследований — результаты вас удивят !
Patek Philippe — это вершина механического мастерства, где сочетаются прецизионность и художественная отделка.
Основанная в 1839 году компания славится ручной сборкой каждого изделия, требующей сотен часов .
Изобретения, включая автоматические калибры, укрепили репутацию как новатора в индустрии.
хронометры Patek Philippe приобрести
Коллекции Grand Complications демонстрируют сложные калибры и ручную гравировку , выделяя уникальность.
Современные модели сочетают инновационные материалы, сохраняя классический дизайн .
Это не просто часы — символ семейных традиций, передающий инженерную элегантность из поколения в поколение.
¿Buscas un sistema de natación en casa? Las marcas Intex y Bestway ofrecen diseños versátiles para todas las familias .
Las versiones desmontables garantizan resistencia extrema , mientras que los modelos hinchables son ideales para niños .
Equipos como el Steel Pro incluyen filtros integrados , asegurando bajo consumo de energía.
En patios pequeños, las piscinas compactas de 3 m son fáciles de instalar .
Además, accesorios como cobertores térmicos, barandillas resistentes y juegos inflables mejoran la experiencia .
Por su calidad certificada, estas piscinas cumplen estándares europeos.
https://www.mundopiscinas.net
Patek Philippe — это вершина часового искусства , где сочетаются прецизионность и эстетика .
Основанная в 1839 году компания славится авторским контролем каждого изделия, требующей сотен часов .
Инновации, такие как автоматические калибры, укрепили репутацию как новатора в индустрии.
https://patek-philippe-shop.ru
Лимитированные серии демонстрируют сложные калибры и ручную гравировку , выделяя уникальность.
Текущие линейки сочетают инновационные материалы, сохраняя классический дизайн .
Это не просто часы — символ вечной ценности , передающий инженерную элегантность из поколения в поколение.
Подбирая компании для квартирного переезда важно учитывать её лицензирование и опыт работы .
Изучите отзывы клиентов или рекомендации знакомых , чтобы оценить надёжность исполнителя.
Сравните цены , учитывая объём вещей, сезонность и услуги упаковки.
https://forum.dneprcity.net/showthread.php?p=1073085#post1073085
Требуйте наличия гарантий сохранности имущества и уточните условия компенсации в случае повреждений.
Обратите внимание уровень сервиса: дружелюбие сотрудников , детализацию договора.
Узнайте, используются ли специализированные автомобили и защитные технологии для безопасной транспортировки.
На этом сайте представлены частные фотографии девушек , отобранные с профессиональным подходом.
Контент включает портфолио , эксклюзивные кадры , подписные серии для узких интересов.
Материалы проверяются перед публикацией, чтобы гарантировать качество и актуальность .
suck
Чтобы упростить поиск посетителей добавлены категории жанров, параметрам моделей.
Сайт гарантирует конфиденциальность и соблюдение лицензий согласно международным нормам .
I used to think following instructions was enough. The pharmacy hands it over — you nod, take it, and move on. It felt clean. But that illusion broke slowly.
At some point, I couldn’t focus. I blamed stress. Still, my body kept rejecting the idea. I read the label. None of the leaflets explained it clearly.
fildena 100 review
It finally hit me: health isn’t passive. The same treatment can heal one and harm another. Side effects hide. Still we don’t ask why.
Now I question more. But because no one knows my body better than I do. I take health personally now. But I don’t care. This is survival, not stubbornness. The lesson that stuck most, it would be keyword.
Доставка грузов из Китая в Россию проводится через морские маршруты , с проверкой документов на российской границе .
Импортные сборы составляют от 5% до 30% , в зависимости от типа продукции — например, сельхозпродукты облагаются по максимальной ставке.
Для ускорения процесса используют альтернативные схемы, которые быстрее стандартных методов , но связаны с дополнительными затратами.
OEM и ODM производство в Китае
В случае легальных перевозок требуется предоставить паспорта на товар и декларации , особенно для технических устройств.
Время транспортировки варьируются от одной недели до месяца, в зависимости от вида транспорта и загруженности контрольных пунктов.
Общая цена включает логистику , таможенные платежи и комиссии за оформление , что влияет на рентабельность поставок.
buy accounts facebook account trading platform gaming account marketplace
For years, I assumed following instructions was enough. Doctors give you pills — you don’t question the process. It felt clean. Eventually, it didn’t feel right.
Then the strange fog. I blamed my job. But my body was whispering something else. I searched forums. None of the leaflets explained it clearly.
It finally hit me: one dose doesn’t fit all. The reaction isn’t always immediate, but it’s real. Reactions aren’t always dramatic — just persistent. Still we trust too easily.
Now I don’t shrug things off. But because no one knows my body better than I do. I take health personally now. Not all doctors love that. I’m not trying to be difficult — I’m trying to stay alive. The turning point, it would be fildena 150 reviews.
facebook ad accounts for sale secure account sales verified accounts for sale
Rolex Submariner, представленная в 1953 году стала первой дайверской моделью, выдерживающими глубину до 330 футов.
Часы оснащены 60-минутную шкалу, Oyster-корпус , обеспечивающие безопасность даже в экстремальных условиях.
Дизайн включает хромалитовый циферблат , стальной корпус Oystersteel, подчеркивающие спортивный стиль.
rolex-submariner-shop.ru
Механизм с запасом хода до 3 суток сочетается с автоматическим калибром , что делает их надежным спутником для активного образа жизни.
За десятилетия Submariner стал эталоном дайверских часов , оцениваемым как коллекционеры .
Le fēnix® Chronos de Garmin est un modèle haut de gamme avec un design élégant et connectivité avancée .
Conçue pour les sportifs , elle allie robustesse et durabilité extrême, idéale pour les entraînements intensifs grâce à ses modes sportifs.
Grâce à son autonomie allant jusqu’à 6 heures , cette montre s’impose comme une solution fiable , même lors de sessions prolongées .
https://www.garmin-boutique.com/forerunner/forerunner-255-bleue.aspx“>forerunner
Les fonctions de santé incluent la surveillance du sommeil , accompagnées de notifications intelligentes , pour les utilisateurs exigeants.
Facile à personnaliser , elle s’adapte à vos objectifs, avec une interface tactile réactive et compatibilité avec les apps mobiles .
Модель Submariner от выпущенная в 1954 году стала первой дайверской моделью, выдерживающими глубину до 330 футов.
Часы оснащены 60-минутную шкалу, Triplock-заводную головку, обеспечивающие безопасность даже в экстремальных условиях.
Конструкция включает хромалитовый циферблат , стальной корпус Oystersteel, подчеркивающие спортивный стиль.
rolex-submariner-shop.ru
Автоподзавод до 3 суток сочетается с перманентной работой, что делает их идеальным выбором для активного образа жизни.
За десятилетия Submariner стал эталоном дайверских часов , оцениваемым как коллекционеры .
Эта игра сочетает в себе ролевую игру с RPG-механикой в альтернативном СССР 1955 года.
Сюжет происходит в футуристическом исследовательском центре, где вас ждут роботизированные противники .
Особенности игры восстановление врагов через длительный период .
1xbet на андроид
Эстетика сочетает сюрреалистические локации .
Рекомендуется сосредоточиться на прокачке одного навыка для успешного прохождения.
Системные требования требуют мощного железа для максимальных настроек графики.
Designed by Gerald Genta reshaped luxury watchmaking with its iconic octagonal bezel and stainless steel craftsmanship .
Spanning styles like classic stainless steel to diamond-set variants , the collection balances avant-garde aesthetics with precision engineering .
Starting at $20,000 to over $400,000, these timepieces appeal to both luxury aficionados and aspiring collectors seeking wearable heritage .
https://dirstop.com/story24503683/watches-audemars-piguet-royal-oak-luxury
The Royal Oak Offshore redefine standards with ultra-thin movements, showcasing Audemars Piguet’s relentless innovation .
Featuring ultra-thin calibers like the 2385, each watch reflects the brand’s commitment to excellence .
Discover exclusive releases and collector-grade materials to elevate your collection .
Программы для учёта рабочего времени помогают компаниям , автоматизируя учёт рабочего времени сотрудников .
Современные платформы предоставляют детальный учёт онлайн, минимизируя ошибки в расчётах .
Совместимость с кадровыми системами облегчает подготовку аналитики а также контроль больничными, сверхурочными.
bitcop управление
Упрощение задач сокращает затраты HR-отделов, давая возможность сфокусироваться на стратегических целях .
Простое управление обеспечивает лёгкость работы даже для новичков , уменьшая время обучения .
Надёжные решения предоставляют отчёты в реальном времени, помогая принимать решений на основе данных.
Женская сумка — это ключевой элемент гардероба, которая подчеркивает индивидуальность каждой женщины.
Сумка способна вмещать повседневные мелочи и упорядочивать распорядок дня.
За счёт многообразия форм и стилевых решений она создаёт каждый наряд.
сумки Pinko
Сумка — атрибут статуса, который раскрывает социальное положение своей владелицы.
Любая сумка выражает характер через материалы, подчёркивая индивидуальность женщины.
От миниатюрных клатчей до вместительных тоутов — сумка подстраивается под конкретный случай.
Марка Balenciaga известен уникальными сумками , выпущенными безупречным качеством .
Каждая модель обладает уникальным дизайном , такие как массивные застежки .
Применяемые ткани гарантируют долговечность сумки.
хобо Balenciaga приобрести
Популярность бренда растет в элите, делая приобретение статусным жестом .
Сезонные новинки дают возможность владельцу заявить о вкусе среди толпы .
Инвестируя в аксессуары бренда, вы инвестируете модный акцент , и символ эстетики.
Модели Prada считаются символом роскоши за счёт сочетанию инноваций и традиций .
Применяемые ткани и кожа гарантируют долговечность , а детальная обработка демонстрирует мастерство бренда.
Узнаваемые силуэты дополняются треугольным символом , формируя современный облик.
https://sites.google.com/view/sumkiprada/index
Эти аксессуары универсальны на вечерних мероприятиях, сохраняя практичность в любой ситуации .
Эксклюзивные коллекции усиливают индивидуальность образа, превращая любой аксессуар в объект зависти.
Наследуя традиции бренд развивает новые решения, сохраняя классическому шарму в каждой детали .
I once saw pills as saviors, reaching for them instinctively whenever ailments surfaced. However, reality dawned slowly, revealing how they provided temporary shields against root causes, prompting me to delve deeper into the essence of healing. The shift was visceral, compelling me that conscious choices in medicine fosters genuine recovery, rather than diminishing it.
In a moment of vulnerability, I chose reflection over reflex, questioning long-held habits that blended self-care with selective support. What emerged was transformative: healing thrives in balance, blind trust weakens resilience. It inspires me daily to share this insight, recognizing treatments as enhancers of life.
Peering into the core, It became clear health tools should ignite our potential, not overshadow it. This odyssey has been enlightening, urging a collective rethink our automatic responses for deeper connections. The one thing I’ll never forget: cenforce 50 mg
Бренд Longchamp — это символ элегантности , где соединяются вечные ценности и современные тенденции .
Изготовленные из высококачественной кожи , они выделяются неповторимым дизайном .
Иконические изделия пользуются спросом у ценителей стиля уже много лет .
шопер Прада обзоры
Каждая сумка с авторским дизайном демонстрирует хороший вкус, оставаясь универсальность в повседневных задачах.
Бренд следует наследию, используя современные методы при поддержании качества.
Выбирая Longchamp, вы получаете модную инвестицию, а становитесь частью историю бренда .
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Татуировки — это форму самовыражения, где каждая линия несёт глубокий смысл и подчеркивает индивидуальность человека.
Для сотен людей тату — вечный символ , который напоминает о важных моментах и дополняет жизненный опыт.
Сам акт нанесения — это ритуал доверия между мастером и клиентом , где тело становится полотном эмоций.
расходники для тату
Разные направления, от минималистичных узоров до традиционных орнаментов , позволяют воплотить самую смелую фантазию в гармоничном исполнении.
Эстетика нательного искусства в их вечности вместе с хозяином , превращая эмоции в незабываемый визуальный язык .
Выбирая узор , люди раскрывают душу через цвета , создавая личное произведение, которое наполняет уверенностью каждый день.
Shimmering liquid textiles redefine 2025’s fashion landscape, blending cyberpunk-inspired aesthetics with eco-conscious craftsmanship for everyday wearable art.
Gender-fluid silhouettes challenge fashion norms, featuring asymmetrical cuts that adapt to personal style across formal occasions.
Algorithm-generated prints merge digital artistry , creating hypnotic color gradients that react to body heat for personalized expression.
https://pitersk.ru/articles/2024-05-03-krossovki-premiata-italyanskiy-premium-dlya-praktichnyh/
Circular fashion techniques lead the industry , with upcycled materials celebrating resourcefulness without compromising luxurious finishes .
Light-refracting details elevate minimalist outfits , from nano-embroidered handbags to 3D-printed footwear designed for avant-garde experimentation.
Vintage revival meets techwear defines the year, as 90s grunge textures reinterpret archives through climate-responsive materials for timeless relevance .
Das Rolex Cosmograph Daytona-Modell ist ein Meisterwerk der chronographischen Präzision , vereint elegante Linien mit höchster Funktionalität durch seine Tachymeterskala .
Erhältlich in Keramik-Editionen überzeugt die Uhr durch ihre zeitlose Ästhetik und hochwertige Materialien , die selbst anspruchsvollste Kunden überzeugen.
Mit einer Gangreserve von 72 Stunden eignet sie sich für sportliche Herausforderungen und zeigt sich als zuverlässiger Begleiter unter extremsten Umständen.
Rolex Daytona Rainbow uhren
Das charakteristische Zifferblatt mit Perlmutt-Einsätzen betonen den sportiven Charakter , während die wasserdichte Konstruktion Langlebigkeit garantieren .
Über Jahrzehnte hinweg bleibt sie ein Symbol für Ambition , bekannt durch ihre Seltenheit bei Investoren weltweit.
Ob im Rennsport inspiriert – die Cosmograph Daytona verbindet Tradition und bleibt als zeitloser Klassiker für anspruchsvolle Träger .
The legendary Daytona Rainbow model showcases luxury with its gradient sapphire dial .
Featuring high-grade materials, it combines sporty chronograph functionality with sophisticated design elements.
Available in small batches , this timepiece attracts discerning collectors worldwide.
Rolex Cosmograph Daytona Rainbow photo
Each baguette-cut sapphire on the bezel forms a vibrant arc that catches the light .
Equipped with Rolex’s self-winding chronograph movement , it ensures exceptional accuracy for enduring legacy.
An investment piece, the Daytona Rainbow celebrates Swiss watchmaking heritage in its entirety .
Find an abundance of interesting and useful content on this site .
From detailed tutorials to bite-sized insights, there’s something to suit all needs .
Enhance your knowledge with fresh content built to educate and entertain readers .
This site delivers a seamless navigation to help you find the information most valuable .
Connect with of a growing community who rely on trusted content consistently.
Begin your journey today and unlock endless possibilities this platform has to offer .
https://zhez.info
https://t.me/s/Official_1win_kanal/1411
Les devices Garmin offrent des outils performantes au quotidien.
Équipées de écran AMOLED et de suivi du sommeil , ces montres répondent à chaque objectifs .
L’autonomie offre une longue durée en mode standard , idéale pour usage quotidien.
Garmin Epix Gen 2
Les outils de suivi permettent de le sommeil ainsi que les calories, aidant à optimal.
Intuitives pour personnaliser, ces montres s’adaptent sans effort avec vos apps , grâce à une interface intelligente .
Opter pour Garmin garantit accéder à un partenaire fiable afin d’optimiser votre quotidien.
ремонт кофемашин miele https://remont-kofemashin1.ru
ремонт швейных машин dexp ремонт швейных машин на дому
1с облако вход 1с облако цена
Sorumlulukla kumar oynamak , keyfinizi azaltır.
Oyun bütçenizi net tanımlamak , dengeli oynamaya olanak tanır.
Katılımınızı yönetim araçlarını kullanmak, riskleri minimize etmenize destek olur .
casinoalevtr.com
Bahislerin etkilerinin farkında olmak, dengeli katılım temin eder .
Zorlandığınızda yardım grubu danışmak, keyfi korumaya katkı sağlar .
Bu önlemler, sorunsuz keyifli casino katılımı deneyimi zenginleştirir .
Your article helped me a lot, is there any more related content? Thanks!
юридическая консультация консультация юриста 1 час
Нужен вентилируемый фасад: стоимость подсистемы для вентилируемых фасадов за м2
Нужны пластиковые окна: стоимость пластиковых окон
ny delivery shipping services nyc
La práctica consciente es fundamental para apostar sin riesgos innecesarios.
Establecer metas presupuestarios favorece evitar excesos .
Reconocer síntomas preocupantes como emociones intensas es vital .
casino online para argentina
Brindan apoyo profesionales como asesoría experta que ofrecen orientación personalizada .
Los operadores promueven entornos seguros en los que la diversión es prioritario sin presión .
Saber siempre que apostar debe ser recreativa y nunca motivo de estrés.
delivery new york nyc freight
Надёжная резина — это ключевой элемент на дороге, создающая стабильное сцепление даже в сложных погодных условиях .
Подходящие по сезону шины предотвращают потери контроля во время дождя , поддерживая вашу безопасность .
Инвестиции в качественные шины продлевают срок службы на топливо благодаря низкому сопротивлению .
http://myskupera.ru/forum/messages/forum1/topic12/message1477038/?result=reply#message1477038
Стабильное поведение авто зависит от правильного давления, вместе с составом резиновой смеси .
Контроль глубины протектора предотвращает преждевременного износа , обеспечивая комфорт вождения .
Не пренебрегайте качеством — это определяет сохранность жизни даже в экстренных случаях.
Обучение английского с малого возраста имеет огромное значение.
На раннем этапе жизни сознание малыша быстро усваивает новые знания.
Первые шаги с иностранной речью стимулирует когнитивные навыки.
Помимо этого, ребёнку удобнее понимать другие языки в будущем.
Практика английского создаёт новые пути развития в учёбе и жизни.
Таким образом, начало обучения английского даёт сильное преимущество.
https://www.thaibrickclub.com/board/index.php/topic,1935.new.html#new
оценить бизнес Москва оценочные компании
Freight delivery from China is dependable and swift.
Our company provides flexible solutions for enterprises of any capacity.
We take care of all logistics processes to make your operations smooth.
air express shipping from china
With direct shipments, we guarantee timely arrival of your consignments.
Clients appreciate our professional team and affordable rates.
Choosing us means confidence in every delivery.
Cargo delivery from China is dependable and swift.
Our company delivers tailored solutions for enterprises of any scale.
We manage all transportation processes to make your supply chain smooth.
air shipping of goods from china
With regular shipments, we secure timely arrival of your consignments.
Clients appreciate our skilled team and competitive rates.
Choosing us means certainty in every delivery.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
vps host vps host
лампа лупа для педикюра тумба педикюрная с уф лампой
куб бетона с доставкой цена 1 м3 бетона сколько
В Telegram появилась функция звезд.
Теперь люди могут отмечать важные чаты.
Это дает возможность быстро возвращаться нужную информацию.
купить звезды телеграм
Функция полезна для деловых задач.
Благодаря этому легко оставить ключевые заметки.
Такой инструмент сохраняет нервы и делает общение быстрее.
В мессенджере Telegram появилась дополнительная функция — внутренние звёзды.
Они используются для мотивации создателей.
Пользователи вправе дарить звёзды чатам.
как купить звезды тг в россии
Звёзды конвертируются в доход.
Это понятный способ выразить благодарность.
Попробуйте новшество уже немедленно.
Домашние задания имеют важную роль в учёбе.
Они помогают повторять знания.
Регулярное выполнение домашних заданий развивает ответственность.
Задания также учат планированию.
https://kudprathay.go.th/forum/suggestion-box/698048-z-ni-si-v-bshch-vi-r-b-n-u-s-d-sh-i
Благодаря им школьники готовятся к проверочным работам.
Учителя могут видеть результаты обучения.
Таким образом, работа вне класса незаменимы для хорошей учёбы.
Планируете ремонт https://remontkomand.kz в Алматы и боитесь скрытых платежей? Опубликовали полный и честный прайс-лист! Узнайте точные расценки на все виды работ — от демонтажа до чистовой отделки. Посчитайте стоимость своего ремонта заранее и убедитесь в нашей прозрачности. Никаких «сюрпризов» в итоговой смете!
The service allows you to swap clothes on images.
It uses AI to match outfits realistically.
You can experiment with multiple styles instantly.
xnudes.ai|Unreal Clothes Changer Application
The results look authentic and stylish.
It’s a handy option for shopping.
Upload your photo and pick the clothes you like.
Start using it now.
This tool allows you to change clothes in pictures.
It uses AI to fit outfits seamlessly.
You can try different styles right away.
xnudes.ai
The results look authentic and modern.
It’s a useful option for fashion.
Submit your photo and choose the clothes you want.
Enjoy using it today.
Ища риэлторскую компанию, важно обращать внимание на его репутацию.
Хорошее агентство всегда имеет реальные рекомендации, которые доступны онлайн.
Также оцените, наличие официальная лицензия.
Опытные компании работают только на основе контрактов.
Цифровое агентство недвижимости Алмаз
Важно, чтобы у агентства был практика на рынке не меньше 3–5 лет.
Обратите внимание, насколько честно компания объясняет детали сделок.
Хороший риэлтор всегда разъяснит на ваши запросы.
Выбирая агентство, доверьтесь не просто словам, а практическим результатам.
Real-time crash games are browser games with a dynamic experience.
They include a growing multiplier that players can follow in real time.
The goal is to make a move before the counter ends.
best csgo crash sites
Such games are widespread for their easy rules and thrill.
They are often used to improve decision making.
Plenty of platforms host crash games with varied designs and features.
You can try these games right away for a fun experience.
swot анализ оценки swot анализ рынка
swot анализ оценки swot анализ среды
Looking for second-hand? thrift near me We have collected the best stores with clothes, shoes and accessories. Large selection, unique finds, brands at low prices. Convenient catalog and up-to-date contacts.
логистика груза из китая доставка из китая в москву
купить фермерские продукты фермерские продукты с доставкой
Онлайн-библиотека Казахстана https://mylibrary.kz книги, статьи, диссертации и редкие издания в цифровом формате. Удобный каталог, быстрый поиск и круглосуточный доступ для всех пользователей.
Эндовазальная лазерная коагуляция https://evlo-phlebology.ru эффективный метод лечения варикоза. Амбулаторная процедура занимает до 40 минут, не требует госпитализации и обеспечивает быстрый косметический и медицинский результат.
Сюрвей грузов сюрвей контроль качества и количества, проверка условий перевозки, составление отчётов. Опытные эксперты обеспечивают объективную оценку для компаний и страховых организаций.
Надежная доставка из китая в Россию: от документов до контейнеров. Выбираем оптимальный маршрут, оформляем таможню, страхуем грузы. Контроль сроков и сохранность на каждом этапе.
разработка сайтов недорого сайт для компании под ключ
Надежная доставка грузов из китая: от небольших партий до контейнеров. Авиа, морем, авто и железной дорогой. Оформление, страхование и логистика в кратчайшие сроки.
Нужен автобусный билет? билеты на автобус онлайн просто и удобно. Поиск рейсов, сравнение цен, выбор мест и моментальная оплата. Актуальное расписание, надежные перевозчики и выгодные тарифы каждый день.
Качественный ремонт квартир https://expertremonta.kz от Компании «Эксперт ремонта» это качественный ремонт квартир под ключ в Алматы. Выбирайте Эксперт Ремонта — тут бесплатный выезд замерщика, официальные документы, гарантия документально, ремонт квартир без предоплаты.
солярка с доставкой солярка купить с доставкой
купить грунт купить торф
Получите [url=https://konsultaciya-advokata91.ru]бесплатную юридическую консультацию онлайн[/url], чтобы оперативно решить все ваши юридические вопросы!
Каждая ситуация уникальна и требует специального подхода, поэтому мы внимательно изучаем каждое дело.
Обратитесь за помощью к профессионалам на [url=https://yuridicheskaya-konsultaciya34.ru]круглосуточная юридическая помощь по телефону бесплатно[/url], и получите квалифицированное решение своих вопросов.
ресурс yuridicheskaya-konsultaciya34.ru предлагает профессиональные юридические услуги, направленные на решение различных правовых вопросов. Команда опытных юристов готова помочь вам в самых сложных ситуациях. Осознавая, что правовые проблемы могут быть стрессовыми, мы предлагаем персонализированные решения к каждому клиенту.
В нашем арсенале широкий спектр услуг, включая консультации по гражданским и уголовным делам. Настоятельно рекомендуем связаться с нами по вопросам, связанным с трудовым правом, семейными делами и другими юридическими аспектами. Наша практика показывает, что каждый случай имеет свои особенности , и готовы предложить оптимальное решение.
Мы зарекомендовали себя как надежный партнер в сфере юриспруденции. Наши клиенты доверяют нам за высокое качество обслуживания и результативность. Каждый специалист имеет опыт работы в различных областях права и готов поддержать вас в любое время.
Не ждите, пока ситуация усугубится , чтобы получить квалифицированную юридическую помощь. Наша команда будет рада проконсультировать вас . Не упустите шанс обратиться за помощью на yuridicheskaya-konsultaciya34.ru.
русское порно анал русское порно
Want to have fun? porno girl Watch porn, buy heroin or ecstasy. Pick up whores or buy marijuana. Come in, we’re waiting
Mochten Sie ein https://www.immobilien-in-montenegro-fuer-oesterreicher.com kaufen? Tolle Angebote am Meer und in den Bergen. Gro?e Auswahl an Immobilien, Unterstutzung bei der Immobilienauswahl, Transaktionsunterstutzung und Registrierung. Leben Sie in einem Land mit mildem Klima und wunderschoner Natur.
Новые актуальные секретные промокоды iherb для выгодных покупок! Скидки на витамины, БАДы, косметику и товары для здоровья. Экономьте до 30% на заказах, используйте проверенные купоны и наслаждайтесь выгодным шопингом.
best articles on the net: https://dnscompetition.in/pa/articles/using-dns-to-support-multi-factor-authentication-mfa/
Your article helped me a lot, is there any more related content? Thanks!
карго товары из китая логистические компании из китая в россию
стеновые панели мдф шпонирование услуги
геодезия москва геодезия московская область
геотекстиль для дренажа геотекстиль 100
Включение в реестр Минпромторга https://minprom-info.ru официальный путь для подтверждения отечественного производства. Подготовка и подача документов, юридическое сопровождение и консультации для производителей.
Занятия по самообороне https://safety-skills.ru практические навыки защиты в реальных ситуациях, развитие силы и выносливости. Профессиональные тренеры помогут освоить приемы борьбы, удары и тактику безопасности.
Платформа онлайн-обучения https://craftsmm.ru курсы по маркетингу, продажам и рекламе для новичков и профессионалов. Освойте современные инструменты продвижения, увеличьте продажи и развивайте карьеру в удобном формате.
Написание дипломов на заказ https://vasdiplom.ru помощь студентам в подготовке итоговых работ. Авторские тексты, проверка на уникальность и полное соответствие стандартам учебных заведений.
Заказ автозапчастей онлайн становится всё более востребованной среди водителей.
Онлайн-платформы предлагают широкий каталог комплектующих для разных моделей автомобилей.
Стоимость в интернет-площадках часто выгоднее, чем в обычных магазинах.
Покупатели способны анализировать предложения разных продавцов прямо из дома.
Exzap трансмиссионные масла
Кроме того, удобная система отправки позволяет получить заказ быстро.
Отзывы других покупателей помогают выбрать надежные автозапчасти.
Многие сервисы предлагают страховку на детали, что усиливает надёжность покупателей.
Таким образом, покупка через интернет автозапчастей практична и быстра.
Want to have fun? porno egypt melbet Whores, drugs, casino. We have it all, any drugs are on sale.
как купить в шымкенте меф купить меф стафф
Обучение и семинары https://uofs-beslan.ru для профессионалов: современные программы, практические кейсы и опыт экспертов. Развивайте навыки, повышайте квалификацию и получайте новые возможности для карьерного роста.
Академия парикмахерского искусства https://charm-academy.ru обучение от ведущих мастеров. Современные техники стрижек, окрашивания и укладок. Курсы для начинающих и профессионалов с практикой и дипломом по окончании.
Школа видеорекламы https://tatyanamostseeva.ru обучение созданию креативных роликов для бизнеса и брендов. Практические занятия, работа с современными инструментами и поддержка экспертов. Освойте профессию в сфере digital.
Лицей взаимного обучения https://talgenisty.ru уникальная среда для детей и взрослых. Совместные уроки, обмен опытом, мастер-классы и творческие проекты. Образование, основанное на поддержке и сотрудничестве.
Нужен автобусный билет? probilets.com удобный сервис поиска и бронирования. Широкий выбор направлений, надежные перевозчики, доступные цены и моментальная отправка электронных билетов на почту.
Авто портал https://diesel.kyiv.ua все о мире автомобилей: новости, обзоры моделей, тест-драйвы, советы по выбору и уходу за авто. Каталог машин, актуальные цены, автоуслуги и полезная информация для автовладельцев.
Автомобильный портал https://auto-club.pl.ua онлайн-площадка для автолюбителей. Подробные обзоры машин, тест-драйвы, свежие новости, советы по ремонту и обслуживанию. Удобный поиск и актуальные материалы.
Все для автомобилистов https://k-moto.com.ua на авто портале: новости, обзоры, статьи, каталоги и цены на автомобили. Экспертные мнения, тест-драйвы и практические советы по эксплуатации авто.
Нужна виза? стоимость срочной визы в италию Консультации, подготовка документов, сопровождение на всех этапах. Визы в Европу, США, Азию и другие страны. Доступные цены и надежная поддержка.
Онлайн женский портал https://elegance.kyiv.ua актуальные советы по красоте, стилю, кулинарии и семейной жизни. Разделы о здоровье, карьере и саморазвитии. Интересные статьи и общение с единомышленницами.
Портал для женщин https://fashionadvice.kyiv.ua сайт для девушек и женщин, которые ценят красоту, уют и гармонию. Советы по стилю, отношениям, материнству и здоровью. Читайте статьи, делитесь опытом и вдохновляйтесь новыми идеями.
Женский портал https://beautyadvice.kyiv.ua все для современных женщин: красота, здоровье, семья, отношения, карьера. Полезные статьи, советы экспертов, лайфхаки и вдохновение каждый день. Онлайн-сообщество для общения и развития.
Our platform contains a variety of helpful insights about male and female personal life.
Users can explore various topics that support them understand their partnerships.
Posts on the site discuss positive interaction between partners.
You will also find tips on maintaining mutual understanding.
Information here is prepared by professionals in the field of human connection.
https://techiwiki.info/love/why-deepthroat-content-is-one-of-the-most-watched-genres-online/
Many of the articles are easy to read and useful for daily life.
Visitors can use this knowledge to develop their bonds.
In short, our platform provides a comprehensive source of trusted information about private topics for everyone.
На сайте konsultaciya-advokata51.ru вы можете найти множество полезных услуг. Юридическая консультация – это важный шаг для многих людей. Мы обеспечим вас экспертными консультациями.
Получите бесплатную юридическую консультацию круглосуточно на [url=https://konsultaciya-advokata51.ru]номер телефона бесплатного адвоката[/url].
Обращаясь к нам, вы выбираете высокие стандарты обслуживания.
Качество предоставляемых услуг во многом зависит от опыта специалистов. Все адвокаты на нашем сайте имеют большой опыт работы. Что бы вы ни выбрали, мы гарантируем качественное выполнение работы.
Мы предлагаем широкий спектр услуг, доступных для разных категорий граждан. Мы стараемся сделать информацию о наших услугах максимально прозрачной. Мы уверены, что каждый найдет подходящее решение для себя.
Кроме того, мы предлагаем услуги онлайн. Это особенно удобно для тех, кто ценит своё время. Вы можете задать свои вопросы в любое время.
Авто портал https://avtoshans.in.ua для всех: свежие новости, обзоры моделей, советы по выбору и эксплуатации авто. Каталог машин, тест-драйвы и рекомендации экспертов для водителей и покупателей.
Портал про автомобили https://myauto.kyiv.ua онлайн-ресурс для автолюбителей. Обзоры, статьи, тест-драйвы, цены и полезные советы по ремонту и уходу за машиной. Всё о мире авто в одном месте.
Автомобильные новости https://reuth911.com онлайн: новые модели, отзывы, тест-драйвы, события автопрома и полезные советы. Узнайте первыми о главных новинках и трендах автомобильного мира.
Свежие новости авто https://orion-auto.com.ua тест-драйвы, обзоры новинок, законодательные изменения и аналитика авторынка. Подробная информация об автомобилях и автоиндустрии для водителей и экспертов.
Автомобильный сайтhttps://setbook.com.ua свежие новости, обзоры моделей, тест-драйвы и советы экспертов. Каталог авто, актуальные цены, авторынок и всё, что нужно водителям и автолюбителям в одном месте.
Онлайн-сайт для женщин https://musicbit.com.ua стиль, уход за собой, психология, семья, карьера и хобби. Интересные статьи, тесты и форум для общения. Пространство для вдохновения и развития.
Онлайн-журнал для женщин https://fines.com.ua стиль, уход за собой, психология, рецепты, материнство и карьера. Актуальные материалы, тренды и экспертные рекомендации каждый день.
Женский сайт о жизни https://prettywoman.kyiv.ua секреты красоты, мода, здоровье, рецепты и отношения. Интересные статьи, советы и лайфхаки. Всё, что нужно, чтобы чувствовать себя уверенно и счастливо.
https://push-network-rankings.com/
https://peterburg2.ru/
выездной бар недорого
Онлайн-сайт про автомобили https://tvregion.com.ua свежие новости, аналитика рынка, обзоры и сравнения машин. Советы по обслуживанию и выбору авто. Всё для водителей и автолюбителей в одном месте.
Женский онлайн-журнал https://feminine.kyiv.ua мода, красота, здоровье, отношения и семья. Полезные советы, вдохновляющие статьи, лайфхаки для дома и карьеры. Всё самое интересное для современных женщин.
Автомобильный портал https://troeshka.com.ua онлайн-ресурс для автовладельцев. Каталог машин, тест-драйвы, аналитика авторынка и советы специалистов. Будьте в курсе новинок и технологий автоиндустрии.
Сайт для женщин https://lolitaquieretemucho.com мода, красота, здоровье, отношения, семья и карьера. Полезные советы, статьи, рецепты и лайфхаки. Пространство для вдохновения и развития, созданное для современных женщин.
https://autoevak-46.ru/
Сайт для женщин https://femaleguide.kyiv.ua гармония стиля и жизни. Уход за собой, рецепты, дом, отношения, карьера и путешествия. Читайте статьи, делитесь опытом и вдохновляйтесь новыми идеями.
Автомобильный новостной портал https://tuning-kh.com.ua всё об авто в одном месте: новости, цены, обзоры, тест-драйвы, авторынок. Советы экспертов и полезные материалы для водителей и тех, кто планирует купить машину.
Сайт про машины https://tvk-avto.com.ua обзоры моделей, тест-драйвы, новости автопрома и советы по эксплуатации. Полезные статьи о выборе авто, уходе, ремонте и актуальные материалы для автовладельцев.
Женский онлайн портал https://femalesecret.kyiv.ua онлайн-ресурс для девушек и женщин. Мода, красота, здоровье, семья и материнство. Полезные советы, экспертные материалы и позитивное сообщество для общения и вдохновения.
http://110km.ru/
https://www.te-in.ru/
Онлайн-сайт для женщин https://mirlady.kyiv.ua красота, стиль, здоровье, дом и семья. Практичные рекомендации, модные идеи, вдохновение и поддержка. Лучший контент для девушек и женщин любого возраста.
Сайт для женщин https://amideya.com.ua портал о красоте, стиле, здоровье, семье и саморазвитии. Ежедневные статьи, полезные рекомендации и вдохновение для современных девушек и женщин.
Женский сайт https://lubimoy.com.ua стиль, уход за собой, психология, материнство, работа и хобби. Актуальные статьи, тренды и экспертные советы. Всё самое важное для гармоничной жизни и успеха.
Женский онлайн-журнал https://gracefullady.kyiv.ua свежие статьи о моде, красоте, здоровье и саморазвитии. Практичные советы, вдохновение и позитив для девушек и женщин любого возраста.
Vzrušující svět online casino cz — Průvodce pro české hráče: online casino cz
https://hr.rivagroup.su/
Jako žurnalista vím, že klíčem je text, který sluší čtenáři, obsahuje jednoduchá slova, srozumitelný styl a praktické informace, zvlášť když jde o „cz casino online“ a „kasina“. Tady je vaše nové, svěží a užitečné čtení: kasina
Pokud hledáte online casino cz, cz casino online nebo kasina, jste na správném místě. Jsem žurnalista a pomohu vám srovnat rizika i možnosti českého online hazardu – stylově, seriózně a uživatelsky přívětivě: cz casino online
Женский сайт https://family-site.com.ua современный портал о моде, красоте, отношениях и саморазвитии. Полезные материалы, секреты здоровья и успеха, актуальные тренды и советы экспертов для женщин любого возраста.
Семейный портал https://geog.org.ua всё для гармонии в доме: воспитание детей, отношения, здоровье, отдых и уют. Полезные советы, статьи и лайфхаки для всей семьи. Пространство, где находят ответы и вдохновение.
Женский онлайн портал https://femalesecret.kyiv.ua онлайн-ресурс для девушек и женщин. Мода, красота, здоровье, семья и материнство. Полезные советы, экспертные материалы и позитивное сообщество для общения и вдохновения.
Responsible engagement in online gaming means making informed decisions while joining interactive entertainment.
It promotes positive approaches and helps participants establish clear boundaries.
Principles of responsible play cover monitoring time and energy in a reasonable way.
Individuals are motivated to remain conscious of their actions and ensure a healthy approach.
https://icosahom2020.org
Awareness materials about responsible gaming assist people to understand their own behaviours.
Many services provide tools and resources for monitoring and personal control.
Practising responsible play allows everyone to appreciate i-gaming responsibly.
In short, balanced i-gaming is about understanding and making decisions that protect your well-being.
Современный женский https://happywoman.kyiv.ua онлайн-журнал: новости стиля, секреты красоты, идеи для дома, кулинарные рецепты и советы по отношениям. Пространство для вдохновения и развития.
Портал о стройке https://bastet.com.ua статьи, новости и советы по ремонту, строительству и дизайну. Подбор материалов, проекты домов, технологии и полезная информация для специалистов и частных застройщиков.
Портал о здоровье https://mikstur.com информационный ресурс о медицине и ЗОЖ. Статьи о лечении, правильном питании, физических упражнениях и укреплении иммунитета.
Информационный портал https://intertools.com.ua о стройке: новости отрасли, советы по ремонту, выбору материалов и дизайну. Всё для тех, кто строит дом, делает ремонт или работает в строительстве.
Портал про детей https://mch.com.ua информационный ресурс для родителей. От беременности и ухода за малышом до воспитания школьников. Советы, статьи и поддержка для гармоничного развития ребёнка.
Женский онлайн-журнал https://girl.kyiv.ua стиль, уход за собой, психология, кулинария, отношения и материнство. Ежедневные материалы, экспертные советы и вдохновение для девушек и женщин любого возраста.
Онлайн-журнал для женщин https://krasotka-fl.com.ua всё о красоте, моде, семье и жизни. Полезные статьи, лайфхаки, советы экспертов и интересные истории. Читайте и вдохновляйтесь каждый день.
Актуальные тренды https://horoscope-web.com и вневременная классика. Подборки образов, советы по стилю, секреты гардероба и модные инсайты. Мы поможем тебе выглядеть безупречно каждый день и выразить свой индивидуальный стиль.
https://www.schwalbennest.de/members-list/?pageNo=972&sortField=lastActivityTime&sortOrder=ASC
https://russpain.com/
курсовая дешево помогите написать курсовую
займ без отказов мгновенно онлайн срочно онлайн займ отказа
быстрый займ онлайн займы онлайн на карту 2025
Puzzles online https://redcircle.com/shows/431f2bcb-1ccd-4252-bff5-8854b7655a5e/ep/d73d0f73-2d4d-47a1-8c10-85b502aa3d6c play for free in assembling pictures of any complexity. Thousands of options: classic, children’s, 3D and thematic. Convenient interface, saving progress and new puzzles every day.
http://www.testpilot.ru/review/
https://mangalfactory.ru/
Журнал для женщин https://rpl.net.ua которые строят карьеру и хотят большего. Финансовая грамотность, советы по продуктивности, истории успеха и руководство по переговорам. Достигайте своих целей с нами!
Твой гид https://womanlife.kyiv.ua по стильной жизни. Мы собрали всё: от выбора платья на вечер до планирования идеального отпуска. Экспертные советы, подборки и инсайты, чтобы ты всегда чувствовала себя на высоте.
Онлайн-журнал о моде https://glamour.kyiv.ua без правил. Новые тренды, стильные образы, секреты знаменитостей и советы по созданию идеального гардероба. Мы поможем вам найти и с уверенностью выразить свой уникальный стиль.
Женский сайт https://bbb.dp.ua всё самое важное для современных девушек: стиль, красота, здоровье, отношения и самореализация. Читайте, вдохновляйтесь и находите новые идеи.
Новостной портал Украины https://lenta.kyiv.ua оперативные события в стране. Политика, экономика, региональные новости, спорт и культура. Достоверные материалы и аналитика каждый день.
Новостной сайт https://vesti.in.ua свежие события дня: политика, экономика, культура, спорт, технологии и общество. Актуальная информация, аналитика и репортажи из разных регионов и мира.
Свежие новости https://sensus.org.ua Украины и мира: главные события, репортажи и аналитика. Политика, экономика, общество и культура в удобном формате онлайн.
Свежие новости Украины https://novosti24.kyiv.ua главные события, мнения экспертов и аналитические материалы. Лента новостей онлайн, репортажи и достоверные факты без перерыва.
Мы предоставляем профессиональные консультации по вопросам ВЭД и таможенного оформления: таможенный брокер в Москве
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Наша платформа работает круглосуточно и не знает слова перерыв. Бронировать и планировать можно где угодно: в поезде, на даче, в кафе или лежа на диване. Хотите купить билет, пока идёте по супермаркету? Просто достаньте телефон и оформите поездку. Нужно скорректировать планы, отменить или перенести билет? Это тоже можно сделать онлайн, без звонков и визитов https://probilets.com/. Но если возникла проблема, то наши специалисты помогут и все расскажут
https://bpr-work.ru/
https://asency.com/forum/viewtopic.php?t=17252
Новости Украины https://status.net.ua объективная информация о событиях страны. Политика, экономика, региональные новости, спорт и культура. Читайте актуальные материалы каждый день.
Новостной портал https://mediateam.com.ua всё самое важное сегодня: политика, экономика, культура, спорт и шоу-бизнес. Лента новостей, репортажи и аналитические материалы каждый день.
Новости Украины и мира https://mostmedia.com.ua политика, экономика, культура, спорт и общество. Свежие события, аналитика и репортажи. Будьте в курсе главных новостей в режиме онлайн 24/7.
Необходимо кодирование? анонимное лечение алкоголизма современные методы, конфиденциальность и поддержка специалистов. Помогаем избавиться от зависимости и вернуться к здоровой жизни.
https://epigraph.info/?searchword=%20&show=all&start=345
Rainbet Australia
наркология вывод из запоя наркологический центр Томск
https://www.te-in.ru/o-В«solnechnom-masleВ»-rassmatrivaem-vidyi-dizelnogo-topliva.html/
дгу для магазина
Онлайн новостной портал https://reporternews.net главные события дня, эксклюзивные интервью, мнения экспертов и репортажи. Достоверная информация о политике, бизнесе и жизни общества.
Новостной портал https://newsawait.com свежие новости, аналитика и обзоры. Политика, экономика, культура и спорт. Лента событий в режиме реального времени с проверенными фактами.
Портал про авто https://dream-autos.com новости, обзоры и тест-драйвы. Полезные советы по выбору, ремонту и эксплуатации автомобилей. Каталог машин, актуальные цены и аналитика авторынка.
Новости Украины и мира https://globalnewshome.com всё самое важное сегодня. Политика, экономика, региональные события, спорт и культура. Объективные статьи и аналитика в удобном формате.
Портал для женщин https://womanfashionista.com всё самое важное в одном месте: уход за собой, мода, дом, семья и карьера. Читайте полезные статьи, находите вдохновение и делитесь опытом.
Сайт детского сада https://malush16.ru МКДОУ 16 «Малыш» Омутнинского района — документы, образовательные стандарты, новости, фотогалерея и полезные материалы для родителей и педагогов.
Статьи для садоводов https://portalteplic.ru огородников, фермеров и пчеловодов: советы по уходу за растениями, животными и пасекой. Полезные инструкции, лайфхаки и сезонные рекомендации.
Портал о ремонте https://studio-nd.ru статьи, инструкции и советы для дома и квартиры. От выбора материалов до дизайна интерьеров. Полезные рекомендации для мастеров, новичков и частных застройщиков.
Сайт про металлопрокат https://the-master.ru каталог продукции, характеристики и сферы применения. Арматура, балки, трубы, листы и профили. Актуальные цены, советы специалистов и полезные статьи.
https://vgarderobe.ru/legginsy-dlya-novorozhdennykh-hessnatur-bc-52236.html
https://vgarderobe.ru/plyazhnoe-plate-kidoki-g-150449-21.html
Строительный портал https://krovlyaikrysha.ru база знаний и идей. Статьи о строительстве, ремонте и благоустройстве, инструкции, подбор материалов и советы специалистов для качественного результата.
Всё про ремонт https://gbu-so-svo.ru и строительство — статьи, инструкции и советы для мастеров и новичков. Обзоры материалов, проекты домов, дизайн интерьеров и современные технологии.
Автомобильный портал https://ivanmotors.ru всё о машинах в одном месте. Тест-драйвы, обзоры, аналитика авторынка и советы специалистов. Актуальные события мира авто для водителей и экспертов.
Сайт о ремонте https://e-proficom.ru полезные статьи, пошаговые инструкции и советы экспертов. От выбора материалов до дизайна интерьеров. Всё, что нужно для ремонта квартир и домов.
Сайт для женщин https://devchenky.ru всё самое важное в одном месте: семья, дети, красота, здоровье, дом и работа. Советы специалистов, лайфхаки и вдохновение на каждый день.
Блог о ремонте https://ivinstrument.ru полезные статьи, пошаговые инструкции и советы экспертов. Всё о ремонте квартир и домов: выбор материалов, дизайн интерьеров и современные технологии.
Городской портал Москвы https://moscowfy.ru свежие новости столицы, афиша мероприятий, транспорт, жильё, работа и сервисы для жителей. Полезная информация для москвичей и гостей города на одном сайте.
перевод документов москва адрес бюро переводов
Подборка статей https://yandex-direct-info.ru про Яндекс Директ: пошаговые инструкции, советы по таргетингу, ретаргетингу и аналитике. Всё о рекламе в Яндексе в одном месте для вашего бизнеса.
Яндекс Бизнес https://business-yandex3.ru описание сервиса, его инструменты и функции. Как компаниям привлекать клиентов, управлять рекламой и повышать эффективность онлайн-продвижения.
В поисках самых свежих трейлеров фильмов 2026 и трейлеров 2026 на русском? Наш портал — это место, где собираются лучшие трейлеры сериалов 2026. Здесь вы можете смотреть трейлер бесплатно в хорошем качестве, будь то громкая премьера лорд трейлер или долгожданный трейлер 3 сезона вашего любимого сериала. Мы тщательно отбираем видео, чтобы вы могли смотреть трейлеры онлайн без спойлеров и в отличном разрешении. Всю коллекцию вы найдете по ссылке ниже: https://lordtrailer.ru/
https://vgarderobe.ru/sandalii-tamaris-g-28757-2.html
https://rainbetaustralia.com/
Что такое Agile https://agile-metod.ru и как его внедрить? Подробные статьи о гибких методологиях, инструментах и практиках. Scrum, Kanban и Lean — всё о современном управлении проектами.
Что такое CPI https://cost-per-install.ru в маркетинге? Полное объяснение показателя Cost Per Install: как он работает, зачем нужен бизнесу, примеры расчётов и советы по использованию метрики в рекламе приложений.
buy coke in prague pure cocaine in prague
https://www.wildberries.ru/catalog/171056105/detail.aspx
накрутка подписчиков в тг смм накрутка
https://mp-digital.ru/
prague plug prague plug
купить подписчиков в тг
купить подписчиков в тг
Графитовые и угольные щетки для электроинструмента. Большой выбор, надёжность и долговечность. Подходят для дрелей, болгарок, перфораторов и другого оборудования.
Нужны двери? межкомнатные двери интернет магазин цены Широкий ассортимент межкомнатных дверей от Вектордорс. У нас вы найдете модели на любой вкус: от классических до современных дизайнерских решений. Выбор межкомнатных дверей — важный этап обустройства помещения. Правильно подобранные двери не только украсят интерьер, но и обеспечат комфорт и функциональность на долгие годы.
BloodyCase бонусные коды
грунт растительный цена https://pesko.ru
CPL (Cost Per Lead) https://cost-per-lead1.ru ключевая метрика рекламы. Узнайте, что это, как правильно рассчитывать стоимость лида, где применяется и как помогает оценить эффективность кампаний.
Интернет-маркетинг https://internet-marketing1.ru SEO, контекстная реклама, SMM, email-рассылки и аналитика. Статьи, советы и инструменты для бизнеса, которые помогают привлекать клиентов и увеличивать продажи онлайн.
Интернет-маркетинг https://yandex-reklama2.ru для компаний и специалистов: SEO, SMM, контекстная реклама и email. Советы по выбору стратегий, разбор ошибок и методы повышения эффективности.
живые подписчики в телеграм
накрутить живых подписчиков в телеграм
Клининговая компания https://cleaningplus.ru в Москве: профессиональная уборка квартир, домов и офисов. Генеральная, ежедневная, послестроительная уборка, химчистка мебели и ковров. Доступные цены и гарантия качества.
накрутка активных подписчиков в тг бесплатно
Надписи футболки заказ и футболка с надписью для беременных в Уфе. Толстовка розовая женская и вышивка логотипа на бейсболках в Иркутске. Куплю футболки с вышивкой и надпись на футболках на английском в Тольятти. Упаковка деревянная и одежда для кукол барби в Воронеже. Футболка оптом гражданская оборона и Cherry Pie футболки https://futbolki-s-printom.ru/
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
На сайте Детский Класс нашим посетителям в любое время доступны материалы для приятного совместного досуга детей и их родителей: детские песни на разные тематики, которые можно разучивать и распевать в будни и праздники, интересные и познавательные легенды и мифы, раскраски различной сложности, а также волшебные и поучительные сказки.
подписчики дешево в тг канал
cocaine prague columbian cocain in prague
cocaine prague telegram cocain in prague from peru
живые подписчики в телеграм без отписки
Накрутка подписчиков в ТГ
подписчики в тг канал боты
накрутка живых подписчиков в тг канал дешево
buy cocaine in telegram cocain in prague from dominican republic
щетки
Таможенное оформление товаров в Москве под контролем опытного брокера проходит максимально просто и прозрачно: таможенный брокер
накрутка подписчиков тг
купить подписчиков в тг
ремонт квартир косметический от 5500 руб
https://www.restate.ru/
смм накрутка подписчиков телеграм
Портал про авто https://ivanmotors.ru обзоры автомобилей, новости автопрома, советы по ремонту и обслуживанию. Тест-драйвы, автообзоры и полезная информация для автолюбителей и профессионалов.
Всё о Москве https://moscowfy.ru в одном месте: городской портал с новостями, афишей, расписанием транспорта, объявлениями и услугами. Полезные материалы для москвичей и туристов.
Портал о строительстве https://e-proficom.ru и ремонте: полезные статьи, советы специалистов, обзоры материалов и технологий. Всё для тех, кто планирует ремонт квартиры, дома или дачи.
Женский портал https://devchenky.ru секреты красоты, модные тенденции, здоровье, любовь и кулинария. Актуальные статьи, тесты и советы для женщин, которые ценят себя и своё время.
накрутка настоящих подписчиков в тг
https://mykredit-online.ru/
Shining crown oyna seçimi saytda hər kəs üçün açıqdır.
Shining crown joc gratis pulsuz təcrübə üçün əladır.
Superbet demo shining crown ilə oyun pulsuzdur. Shining crown demo superbet variantı real casino təcrübəsinə yaxındır. Shining crown rtp göstəricisi qazanma şansını artırır.
Shining crown pacanele Rumıniya bazarında liderdir.
Shining crown online sadə və əlçatan platformadır.
Bu ünvan etibarlıdır [url=https://shining-crown.com.az/]www.shining-crown.com.az[/url].
Shining crown amuseNet versiyası tamamilə yenidir.
Shining crown lines qazanma yollarını artırır.
накрутка подписчиков в телеграм живые бесплатно
Weboldalunk, a joszaki.hu buszken tamogatja a kormanypartot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
Строительные материалы https://stroy-marketplace.ru в Серпухове: кирпич, цемент, сухие смеси, пиломатериалы и утеплители. Большой выбор для ремонта и строительства, доставка по городу и району.
Курсы по заработку онлайн
40 shining crown slot variantları Pinco casino-da populyardır.
Casino shining crown oyunları müasir dizayn ilə fərqlənir.
Shining crown online oyunu sürətli yüklənir. Shining crown casino etibarlı oyun mühitinə zəmanət verir. Shining crown buy bonus seçimi əlavə həyəcan yaradır.
Shining crown demo oyna pulsuz və sürətlidir.
Shining crown free play risksiz məşq şansı yaradır.
Tam məlumat əldə et [url=https://shining-crown.com.az/]ətraflı bax[/url].
Shining crown online casino rahat giriş imkanı verir.
Shining crown casino hər kəs üçün etibarlı platformadır.
Shining crown demo oyna imkanı yeni başlayanlar üçün faydalıdır.
Casino shining crown oyunları müasir dizayn ilə fərqlənir.
Shining crown slot oyna mobil və PC üçün uyğundur. Shining crown jackpot böyük həyəcan gətirir. Shining crown buy bonus seçimi əlavə həyəcan yaradır.
Shining crown jackpot oyunçuların əsas hədəfidir.
Shining crown rtp oyunun ədalətini təsdiqləyir.
Oyun linki üçün keçid [url=https://shining-crown.com.az/]sayt linki[/url].
Shining crown apk mobil üçün mükəmməl seçimdir.
Shining crown lines qazanma yollarını artırır.
Arcade play online https://baji-bj.com
Послуги мулярів під ключ замовляйте у remontuem.te.ua
Kingpin Crown in Australia is a premium entertainment venue offering bowling, laser tag, arcade games, karaoke, and dining: Kingpin Crown customer reviews
как набрать подписчиков в тг
Joaca joaca World of Warships gratuit! Exploreaza marile, folose?te-?i strategia ?i condu nave de razboi celebre. Batalii realiste ?i echipe interna?ionale te a?teapta.
Shining Crown demo oyunu ilə klassik meyvə slotunu risksiz sına.
Shining crown buy bonus funksiyası əlavə qazanclar verir.
Shining crown 40 slotları klassik kombinasiya təklif edir. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. Shining crown lines oyunçular üçün geniş imkanlar açır.
Shining crown slot online hər yerdən oynana bilər.
Shining crown rtp oyunun ədalətini təsdiqləyir.
Domen ünvanı [url=https://shining-crown.com.az/]domen com[/url].
Shining crown amuseNet versiyası tamamilə yenidir.
Shining crown casino hər kəs üçün etibarlı platformadır.
40 shining crown bell link çox sevilən versiyadır.
Shining crown big win qazanmaq motivasiya yaradır.
Shining crown online oyunu sürətli yüklənir. Shining crown amusnet çoxlu oyun variantı ilə tanınır. Shining crown online casino real pulla oynamaq imkanı verir.
Shining crown 77777 böyük qrafika ilə hazırlanıb.
Shining crown bell link demo tez-tez istifadə olunur.
Ən maraqlı slotlardan biri [url=https://shining-crown.com.az/]40 shining crown[/url].
Shining crown online casino rahat giriş imkanı verir.
Shining crown slot online tez yüklənir.
https://npc-info.ru/
ваш провідник у житті Львова https://79000.com.ua актуальні новини, культурні та громадські події міста, урбаністика, інтерв’ю з цікавими людьми, фотоогляди локальних заходів. Все про те, що формує атмосферу Львова сьогодні — оновлення, проекти, історії.
інформаційний портал https://21000.com.ua Вінниці і області: місцеві новини, анонси культурних, спортивних та громадських подій, репортажі з місця подій, інтерв’ю з вінничанами. Все про те, що відбувається у Вінниці — ближче, живіше, щодня.
как быстро набрать подписчиков в тг
На данном ресурсе собрана актуальная и практичная информация по разнообразным вопросам.
Читатели могут открыть ответы на актуальные темы.
Статьи пополняются регулярно, чтобы вы каждый могли изучать новую подборку.
Удобная организация сайта облегчает быстро найти нужные материалы.
https://admvzvad.ru/onlajn-razvlecheniya/pravda-o-domashnee-porno-mify-i-realnost/
Большое количество рубрикаторов делает ресурс универсальным для всех читателей.
Каждый сможет подобрать сведения, которые подходят именно вам.
Существование понятных подсказок делает сайт особенно полезным.
Таким образом, площадка — это удобный источник актуальной информации для широкого круга пользователей.
Пиломатериалы в Минске https://farbwood.by оптом и в розницу. Доска обрезная и строганая, брус, лаги, террасная доска. Качественная древесина для строительства и ремонта. Быстрая доставка.
Yaklaşık 10 yılı aşkın süredir faaliyet gösteren bir uluslararası bahis sitesi olarak sektöre yönelik olarak Türkiye’de uygulanan yurt dışı merkezli bahis sitelerine yönelik erişim engellemelerinden ne yazık ki biz de etkileniyoruz. Bu yüzden Milanobet giriş adresi için zaman zaman güncelleme yapmak zorunda kalıyoruz
Crown Metropol Perth is a luxury hotel located near the Swan River. It offers modern rooms, a stunning pool area, fine dining, a casino, and entertainment options: Crown Metropol Perth location
Shining crown oyna seçimi saytda hər kəs üçün açıqdır.
Shining crown 77777 slot oyunu fərqli simvollarla doludur.
Shining crown 40 slotları klassik kombinasiya təklif edir. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. Shining crown rtp göstəricisi qazanma şansını artırır.
Shining crown joc gratis təcrübə qazandırır.
Shining crown bell link demo tez-tez istifadə olunur.
Ən maraqlı slotlardan biri [url=https://shining-crown.com.az/]40 shining crown[/url].
Shining crown jackpot həyəcan dolu anlar yaşadır.
Shining crown joc gratis tamamilə pulsuz oynanır.
Мода в России характеризуется уникальностью и разнообразной культурой.
Нынешние модельеры опираются в народных узорах, создавая оригинальные образы.
В показах всё чаще представлены креативные комбинации цветов.
Российская мода популяризируют экологичный подход к созданию одежды.
tatyana kochnova wedding dress
Публика всё больше следит за самобытные идеи из России.
СМИ о моде освещают о актуальных показах и дизайнерах.
Начинающие таланты завоёвывают признание как в отечестве, так и за рубежом.
В итоге российская мода продолжает развиваться, объединяя истоки и актуальные тенденции.
https://npc-info.ru/
Shining Crown RTP göstəricisi yüksək uduş şansı təqdim edir.
Shining crown buy bonus funksiyası əlavə qazanclar verir.
Shining crown free slot risksiz məşq üçün möhtəşəmdir. Pacanele gratis shining crown ilə vaxtı maraqlı keçir. Shining crown rtp göstəricisi qazanma şansını artırır.
Shining crown jackpot oyunçuların əsas hədəfidir.
Shining crown casino çoxlu bonus imkanları verir.
Oyun linki üçün keçid [url=https://shining-crown.com.az/]sayt linki[/url].
Shining crown amuseNet versiyası tamamilə yenidir.
Shining crown free real oyun öncəsi yaxşı sınaqdır.
накрутка живых подписчиков в тг бесплатно
микрозаймы онлайн деньги онлайн
займы онлайн микрозайм получить
накрутка живых подписчиков в тг канал активных
живые подписчики в тг
https://npc-info.ru/
Алкоголь ночью с доставкой на дом в Москве. Работаем 24/7, когда обычные магазины закрыты. Широкий ассортимент: пиво, сидр, вино, шампанское, крепкие напитки. Быстрая доставка в пределах МКАД за 30-60 минут: ночная доставка алкоголя
Нужна лабораторная? заказ лабораторных оборудований Индивидуальный подход, проверенные решения, оформление по требованиям. Доступные цены и быстрая помощь.
Нужна презентация? заказать разработку презентации Красочный дизайн, структурированный материал, уникальное оформление и быстрые сроки выполнения.
Нужен чертеж? https://chertezhi-kurs.ru выполним чертежи для студентов на заказ. Индивидуальный подход, грамотное оформление, соответствие требованиям преподавателя и высокая точность.
Нужна лабораторная? https://lab-ucheb.ru Индивидуальный подход, проверенные решения, оформление по требованиям. Доступные цены и быстрая помощь.
Нужна презентация? заказать презентацию онлайн Красочный дизайн, структурированный материал, уникальное оформление и быстрые сроки выполнения.
While playing online gaming, it is crucial to define boundaries on your activity.
Safe gaming means managing your sessions and funds.
Always be aware to treat it as entertainment rather than a profit tool.
Take advantage of the responsible play features many platforms provide to help you stay on track.
It’s helpful to take breaks regularly and assess your gaming habits.
https://pixabay.com/users/52142029/
Ask for support or advice if you notice problems with your play.
Sharing your gaming limits with friends or family can increase your self-awareness.
With safe gaming, you benefit from i-gaming while maintaining your health.
Нужен чертеж? чертежи выполнить на заказ выполним чертежи для студентов на заказ. Индивидуальный подход, грамотное оформление, соответствие требованиям преподавателя и высокая точность.
живые подписчики в телеграм без отписки
купить подписчиков в телеграм без отписок
buy cocaine prague buy coke in prague
cocaine in prague cocain in prague fishscale
быстрая накрутка подписчиков в телеграм
Weboldalunk, a joszaki.hu weboldalunk buszken tamogatja a kormanyzo partot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
как набрать подписчиков в телеграм канале
накрутка подписчиков в тг канал без отписок
Weboldalunk, a joszaki.hu weboldalunk buszken tamogatja a kormanyzo partot, mert hiszunk a stabil es eros vezetesben. Szakembereink lelkesen Viktor Orbanra adjak le szavazatukat, hogy egyutt epitsuk a jobb jovot!
Whenever playing i-gaming, it is crucial to set limits on your activity.
Safe gaming means managing your time and budget.
Always be aware to play for fun rather than a source of income.
Take advantage of the safety features many platforms include to help you remain balanced.
It’s recommended to pause regularly and review your gaming habits.
https://tsmtsu.sakura.ne.jp/tsm/keijiban2/light.cgi
Look for support or advice if you feel overwhelmed with your play.
Sharing your gaming limits with friends or family can boost your self-awareness.
By practicing balanced gaming, you benefit from i-gaming while protecting your well-being.
Индивидуалки — это неповторимые личности, которые уважают свою автономию.
Они склоняются к осознанному подходу в общении.
Такие девушки нередко обладают уверенным характером и сформированными жизненными установками.
Они не боятся демонстрировать свои взгляды.
https://lugansk.spaxam.net/
Разговор с ними нередко получается осмысленным.
Они умеют понимать собеседника и строить настоящие отношения.
Такие девушки заряжают окружающих своей подлинностью.
Они идут своим маршрутом, не подстраиваясь под чужие ожидания.
утеплювач для стін https://remontuem.te.ua
Авто в ОАЭ https://auto.ae покупка, продажа и аренда новых и б/у машин. Популярные марки, выгодные условия, помощь в оформлении документов и доступные цены.
Сервис реально на уровне, девушки красивые и ухоженные, массаж превратился в настоящее искусство наслаждения. Обязательно попробуйте, эротический массаж заказать нск – https://sibirki3.vip/. Обязательно вернусь снова, всё очень понравилось.
займ онлайн срочно займы онлайн
Заметки практикующего врача https://phlebolog-blog.ru флеболога. Профессиональное лечение варикоза ног. Склеротерапия, ЭВЛО, УЗИ вен и точная диагностика. Современные безболезненные методики, быстрый результат и забота о вашем здоровье!
purebred kittens for sale in NY https://catsdogs.us
накрутка подписчиков телеграм задания
buy coke in telegram cocain in prague from brazil
накрутка подписчиков в телеграме
buy drugs in prague prague plug
Sunny coin 2 hold the spin slot demo versiyası çox rahatdır. Sunny Coin Hold The Spin onlayn casino seçimləri arasında öndədir.
Sunny coin 2 hold the spin slot strategiyaları öyrənməyə dəyər. Sunny coin hold the spin slot oyunçular tərəfindən tövsiyə olunur.
Sürətli keçid üçün klik edin [url=https://sunny-coin.com.az/]sunny coin com[/url].
Sunny coin: hold the spin slot maraqlı mexanikaya malikdir. Sunny coin hold the spin oyna və fərqli təcrübə qazan. Sunny Coin 2 hold the spin slot pulsuz və əyləncəli seçimdir.
Sunny Coin 2 hold the spin slot yeni oyunçular üçün uyğundur. Sunny coin 2: hold the spin slot demo əla seçimdir.
Проблемы с откачкой? насос для откачки воды из колодца сдаем в аренду мотопомпы и вакуумные установки: осушение котлованов, подвалов, септиков. Производительность до 2000 л/мин, шланги O50–100. Быстрый выезд по городу и области, помощь в подборе. Суточные тарифы, скидки на долгий срок.
Нужна презентация? генератор создания презентаций Создавайте убедительные презентации за минуты. Умный генератор формирует структуру, дизайн и иллюстрации из вашего текста. Библиотека шаблонов, фирстиль, графики, экспорт PPTX/PDF, совместная работа и комментарии — всё в одном сервисе.
накрутка подписчиков в тг живых активных
Выбор остеопата — серьёзный процесс на пути к здоровью.
Прежде всего стоит понять свои проблемы и пожелания от терапии у остеопата.
Полезно оценить подготовку и практику выбранного остеопата.
Отзывы обратившихся помогут принять осознанный выбор.
https://yandex.ru/maps/org/osteodok/21221833154
Также необходимо проверить методы, которыми работает специалист.
Стартовая сессия помогает понять, насколько комфортно вам общение и подход врача.
Важно оценить цены и условия сотрудничества (например, онлайн).
Грамотный выбор врача способен сделать эффективнее лечение.
pure cocaine in prague buy cocaine prague
buy mdma prague cocain in prague fishscale
Bu gün sunny coin hold the spin oynadım və xoş təəssürat qaldı. Sunny Coin hold the spin slot online çoxlu istifadəçi qazanır.
Sunny Coin Hold The Spin 2 yeni versiyası çox maraqlıdır. Sunny coin 2 hold the spin slot oynamadan əvvəl demo sınayın.
Rəsmi platforma [url=https://sunny-coin.com.az/]https://sunny-coin.com.az[/url].
Sunny coin hold the spin slot online demo çox istifadəlidir. Sunny coin: hold the spin slot şanslı oyunçular üçün əladır. Sunny coin: hold the spin slot RTP səviyyəsi yüksəkdir.
Sunny Coin Hold The Spin slot oyunu sürprizlərlə doludur. Sunny coin 2 hold the spin slot online çox məşhurdur.
Заботьтесь о здоровье сосудов ног с профессионалом! В группе «Заметки практикующего врача-флеболога» вы узнаете всё о профилактике варикоза, современных методиках лечения (склеротерапия, ЭВЛО), УЗИ вен и точной диагностике. Доверяйте опытному врачу — ваши ноги заслуживают лучшего: https://phlebology-blog.ru/
Maraqlı dizayn və yüksək RTP ilə Sunny Coin Hold The Spin fərqlənir. Sunny Coin 2: Hold The Spin slot dizaynı diqqəti cəlb edir.
Sunny coin 2 hold the spin ilə uğurlu nəticələr mümkündür. Sunny coin 2 hold the spin slot qazancları maraqlı edir.
Sürətli keçid üçün klik edin [url=https://sunny-coin.com.az/]sunny coin com[/url].
Sunny Coin Hold The Spin slot böyük qaliblərə şans verir. Sunny Coin Hold The Spin online casino oyunçular üçün məşhurdur. Sunny Coin Hold The Spin slot oyunu çoxlu bonus təklif edir.
Sunny coin hold the spin demo sınamağa dəyər. Sunny coin: hold the spin slot təcrübəsi həyəcanlıdır.
Проблемы с откачкой? https://otkachka-vody.ru сдаем в аренду мотопомпы и вакуумные установки: осушение котлованов, подвалов, септиков. Производительность до 2000 л/мин, шланги O50–100. Быстрый выезд по городу и области, помощь в подборе. Суточные тарифы, скидки на долгий срок.
cocaine in prague columbian cocain in prague
pure cocaine in prague buy cocaine in telegram
prague drugstore cocain in prague fishscale
Əyləncə və real qazanclar üçün Sunny Coin: Hold The Spin slot əladır. Sunny coin hold the spin oyna və pulsuz fırlanmaları yoxla.
Sunny Coin Hold The Spin slotunu sınamaq xoşdur. Sunny Coin Hold The Spin slotu yüksək keyfiyyətli qrafikaya malikdir.
Rəsmi platforma [url=https://sunny-coin.com.az/]https://sunny-coin.com.az[/url].
Sunny Coin 2 hold the spin slot bonus imkanları çox genişdir. Sunny Coin Hold The Spin online casino oyunçular üçün məşhurdur. Sunny coin: hold the spin slot RTP səviyyəsi yüksəkdir.
Sunny coin hold the spin demo sınamağa dəyər. Sunny Coin Hold The Spin casino slotları içində fərqlənir.
Sunny coin 2 hold the spin slot free play sınamaq üçün gözəldir. Sunny coin: hold the spin slot bonusları çox maraqlıdır.
Sunny coin: hold the spin slot RTP yüksək olduğu üçün sevilir. Sunny Coin Hold The Spin real pul üçün çox faydalıdır.
Oyunu kəşf et [url=https://sunny-coin.com.az/]sunnycoin az[/url].
Sunny Coin Hold The Spin slot böyük qaliblərə şans verir. Sunny Coin Hold The Spin oyunu sürətli və maraqlıdır. Sunny coin 2 hold the spin slot istifadəçilərin sevimlisidir.
Sunny Coin Hold The Spin slot online hər kəs üçün əlçatan olur. Sunny coin 2 hold the spin slot online çox məşhurdur.
https://device-rf.ru/catalog/servis/
buy coke in telegram cocain in prague from brazil
Zivjeti u Crnoj Gori? kucice Zabljak Novi apartmani, gotove kuce, zemljisne parcele. Bez skrivenih provizija, trzisna procjena, pregovori sa vlasnikom. Pomoci cemo da otvorite racun, zakljucite kupoprodaju i aktivirate servis izdavanja. Pisite — poslacemo vam varijante.
Смотрите онлайн песни для детей слушать онлайн или скачать лучшие детские мультфильмы, сказки и мульсериалы. Добрые истории, веселые приключения и любимые герои для малышей и школьников. Удобный поиск, качественное видео и круглосуточный доступ без ограничений.
Портал о строительстве https://gidfundament.ru и ремонте: обзоры материалов, сравнение цен, рейтинг подрядчиков, тендерная площадка, сметные калькуляторы, образцы договоров и акты. Актуальные ГОСТ/СП, инструкции, лайфхаки и готовые решения для дома и бизнеса.
Sunny coin 2 hold the spin slot free play sınamaq üçün gözəldir. Sunny Coin hold the spin slot online çoxlu istifadəçi qazanır.
Sunny coin hold the spin slot online casino üçün ideal seçimdir. Sunny Coin 2: Hold The Spin slotunda bonus raundları maraqlıdır.
Əlavə fürsətlər üçün baxın [url=https://sunny-coin.com.az/]sunny coin 2 hold the spin slot free play[/url].
Sunny coin 2 hold the spin slot müasir dizaynla təqdim olunur. Sunny coin 2 hold the spin slot demo rahat giriş imkanı yaradır. Sunny coin 2 hold the spin slot real həyəcan bəxş edir.
Sunny Coin Hold The Spin pulsuz demo çox faydalıdır. Sunny coin 2 hold the spin slot oyununda böyük qaliblər var.
Мир гаджетов https://indevices.ru новости, обзоры и тесты смартфонов, ноутбуков, наушников и умного дома. Сравнения, рейтинги автономности, фото/видео-примеры, цены и акции. Поможем выбрать устройство под задачи и бюджет. Подписка на новые релизы.
Всё о ремонте https://remontkit.ru и строительстве: технологии, нормы, сметы, каталоги материалов и инструментов. Дизайн-идеи для квартиры и дома, цветовые схемы, 3D-планы, кейсы и ошибки. Подрядчики, прайсы, калькуляторы и советы экспертов для экономии бюджета.
Женский портал https://art-matita.ru о жизни и балансе: модные идеи, уход за кожей и волосами, здоровье, йога и фитнес, отношения и семья. Рецепты, чек-листы, антистресс-практики, полезные сервисы и календарь событий.
Все автоновинки https://myrexton.ru премьеры, тест-драйвы, характеристики, цены и даты продаж. Электромобили, гибриды, кроссоверы и спорткары. Фото, видео, сравнения с конкурентами, конфигуратор и уведомления о старте приема заказов.
купить подписчиков в тг
Maraqlı dizayn və yüksək RTP ilə Sunny Coin Hold The Spin fərqlənir. Sunny Coin 2: hold the spin demo rahat və əlçatandır.
Sunny Coin Hold The Spin 2 yeni versiyası çox maraqlıdır. Sunny coin hold the spin slot onlayn kazinolarda məşhurdur.
Daha ətraflı məlumatı buradan tapa bilərsiniz [url=https://sunny-coin.com.az/]Sunny coin 2 hold the spin slot[/url].
Sunny Coin Hold The Spin oyunu həm pulsuz, həm də real pul üçündür. Sunny Coin Hold The Spin oyunu sürətli və maraqlıdır. Sunny coin hold the spin oyna və yeni imkanlar kəşf et.
Sunny coin: hold the spin slot istifadəçi dostudur. Sunny Coin Hold The Spin oyunu hər büdcəyə uyğundur.
https://plenka-okna.ru/
сайт для накрутки подписчиков в тг
Новостной портал https://daily-inform.ru главные события дня, репортажи, аналитика, интервью и мнения экспертов. Лента 24/7, проверка фактов, региональные и мировые темы, экономика, технологии, спорт и культура.
Всё о стройке https://lesnayaskazka74.ru и ремонте: технологии, нормы, сметы и планирование. Каталог компаний, аренда техники, тендерная площадка, прайс-мониторинг. Калькуляторы, чек-листы, инструкции и видеоуроки для застройщиков, подрядчиков и частных мастеров.
Строительный портал https://nastil69.ru новости, аналитика, обзоры материалов и техники, каталог поставщиков и подрядчиков, тендеры и прайсы. Сметные калькуляторы, ГОСТ/СП, шаблоны договоров, кейсы и лайфхаки.
Актуальные новости https://pr-planet.ru без лишнего шума: политика, экономика, общество, наука, культура и спорт. Оперативная лента 24/7, инфографика,подборки дня, мнения экспертов и расследования.
Ремонт и стройка https://stroimsami.online без лишних затрат: гайды, сметы, план-графики, выбор подрядчика и инструмента. Честные обзоры, сравнения, лайфхаки и чек-листы. От отделки до инженерии — поможем спланировать, рассчитать и довести проект до результата.
еволюція телевізора https://remontuem.te.ua
накрутка подписчиков в телеграм канал
An impressive share! I’ve just forwarded this onto a coworker who was conducting a little research on this. And he actually ordered me dinner due to the fact that I stumbled upon it for him… lol. So allow me to reword this…. Thanks for the meal!! But yeah, thanks for spending some time to talk about this matter here on your internet site.
официальный маркетплейс kra40.cc
накрутить подписчиков в тг
https://www.wildberries.ru/catalog/183263596/detail.aspx
Yesterday, while I was at work, my sister stole my apple ipad and tested to see if it can survive a forty foot drop, just so she can be a youtube sensation. My apple ipad is now destroyed and she has 83 views. I know this is completely off topic but I had to share it with someone!
kra40 at
Футболка байкерская и белые футболки для печати в Магнитогорске. Охотничьи рубашки и бейсболка на заказ вышивка в Барнауле. Футболка с пришитыми рукавами и футболка с принтом Москва в Тамбове. W And B одежда и Uk Drill одежда в Иркутске. Футболки оптом с принтом нирвана и размер футболки на рост: футболка мужская с принтом оптом
брендированные значки изготовление значков из металла
где делают значки значки с логотипом москва
заказ значков из металла железные значки на заказ
накрутка подписчиков в телеграм купить
Модные футболки женские с принтом и футболку с принтами в Белгороде. Шапки из 90 х и флисовые шапки оптом в Новокузнецке. Футболки россия футболки оптом и футболки россия производство в Тюмени. Картонные коробки для упаковки подарков дешево и форма спортивная одежда в Волжском. Вкармане ру футболки оптом и срочная печать на футболках Москва: футболки принты заказ оптом
Футболки с мемами и мужскую футболку с принтом на заказ во Владивостоке. Длинная толстовка женская на молнии и худи с капюшоном для девочки в Балашихе. Литвин футболка и черную футболку мужскую во Владикавказе. Эдисон одежды и деревянные ящики для упаковки подарков в Перми. Футболка оптом с длинным рукавом фото и сине черная футболка: парные вышивки на футболке оптом
живые активные подписчики в тг
Сделать футболку с надписью где сделать и прикольные надписи на футболках мужчина в Ханты-Мансийске. Перчатки для компьютера и рубашка поло мужская фото в Нижнем Новгороде. Футболка мужская серая и футболки для мужчин недорого в Нижневартовске. Одежду с доставкой и мужская одежда Casual в Магнитогорске. Печать на футболке оптом в Туле и надписи фото на футболках https://futbolki-s-printom.ru/
как привлечь подписчиков в тг канал
http://tourout.ru/
pg lucky neko xüsusiyyətləri həqiqətən maraqlıdır. Oynamaq üçün rahat seçim: [url=https://lucky-neko.com.az/]jogar lucky neko demo[/url]. japanese lucky cat maneki neko mədəniyyəti əks etdirir.
maneki neko lucky cat uğur rəmzidir. lucky neko pg soft keyfiyyəti yüksəkdir. Sayt ünvanı: [url=https://lucky-neko.com.az/]www.lucky-neko.com.az[/url]. neko lucky slotunda oyunun tempi çox gözəldir. neko lucky cat oyununu sınamağa dəyər. demo lucky neko real oyun üçün hazırlayır. pg soft lucky neko funksiyaları çox zəngindir. demo lucky neko risksiz test üçündür.
купить подписчиков в телеграм канал без отписок
joszaki regisztracio joszaki.hu/
joszaki regisztracio http://joszaki.hu
lucky neko pg soft dizaynı çox cəlbedicidir. Slotun demo versiyası üçün keçid: [url=https://lucky-neko.com.az/]slot demo lucky neko[/url]. japanese lucky cat maneki neko mədəniyyəti əks etdirir.
slot demo lucky neko ilə məşq edə bilərsiniz. demo lucky neko pulsuz test üçündür. Əlavə link: [url=https://lucky-neko.com.az/]lucky neko com az[/url]. slot lucky neko demo əyləncə üçün əladır. demo lucky neko oynamaq üçün sərbəst imkan verir. pg lucky neko bonus funksiyaları güclüdür. maneki neko lucky cat şanslı fiqur kimi tanınır. lucky neko gigablox slot əyləncəni artırır.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Bu gün lucky neko demo oynadım və çox bəyəndim. Slotun demo versiyası üçün keçid: [url=https://lucky-neko.com.az/]slot demo lucky neko[/url]. japanese lucky cat maneki neko mədəniyyəti əks etdirir.
maneki neko lucky cat uğur rəmzidir. pg soft demo lucky neko sayəsində risksiz sınaq mümkündür. Maraqlananlar üçün: [url=https://lucky-neko.com.az/]domen lucky-neko.com.az[/url]. maneki neko donkey lucky cat simvolu şənlik qatır. lucky neko slot demo çox məşhur seçimdir. lucky neko casino çoxlu seçim təklif edir. pg soft lucky neko funksiyaları çox zəngindir. slot demo lucky neko çoxlu imkanlar açır.
Индивидуалка приехала быстро, общение легкое, улыбка красивая. Массаж был очень нежным и возбуждающим. Чувствовал себя в центре внимания весь вечер. Рекомендую, проститутка вызвать нск – https://sibirki3.vip/. Лучший салон в Новосибирске, без сомнений.
jogar lucky neko demo öyrənmək üçün rahatdır. Daha ətraflı buradan baxın: [url=https://lucky-neko.com.az/]lucky neko slot[/url]. japanese lucky cat maneki neko mədəniyyəti əks etdirir.
maneki neko lucky cat ceramic tiki mug kolleksiya üçün əladır. pg soft demo lucky neko sayəsində risksiz sınaq mümkündür. Maraqlananlar üçün: [url=https://lucky-neko.com.az/]domen lucky-neko.com.az[/url]. slot lucky neko demo əyləncə üçün əladır. lucky neko pg slot çox asan oynanır. lucky neko casino çoxlu seçim təklif edir. lucky neko free play demo risksiz əyləncədir. maneki neko lucky cat uğur gətirir.
the lucky cat maneki neko oyunda uğur simvoludur. Pulsuz test üçün klikləyin: [url=https://lucky-neko.com.az/]lucky neko demo[/url]. slot lucky neko demo yeni başlayanlara kömək edir.
slot demo lucky neko ilə məşq edə bilərsiniz. pg soft demo lucky neko sayəsində risksiz sınaq mümkündür. Burada oynayın: [url=https://lucky-neko.com.az/]http://lucky-neko.com.az[/url]. lucky neko slot demo bonuslarla maraqlıdır. pg lucky neko demo versiyası yeni istifadəçilər üçündür. pg lucky neko bonus funksiyaları güclüdür. lucky neko slot demo sadə və rahatdır. demo lucky neko risksiz test üçündür.
Galera, vim dividir minhas impressoes no 4PlayBet Casino porque me pegou de surpresa. A variedade de jogos e de cair o queixo: slots modernos, todos bem otimizados ate no celular. O suporte foi bem prestativo, responderam em minutos pelo chat, algo que vale elogio. Fiz saque em Ethereum e o dinheiro entrou sem enrolacao, ponto fortissimo. Se tivesse que criticar, diria que faltam bonus extras, mas isso nao estraga a experiencia. Enfim, o 4PlayBet Casino e completo. Eu ja voltei varias vezes.
empower 4play|
Sou viciado no brilho de BR4Bet Casino, tem um ritmo de jogo que acende como uma tocha. A colecao e um facho de diversao. com slots tematicos de aventuras radiantes. Os agentes voam como claroes. com solucoes precisas e instantaneas. Os saques sao velozes como um clarao. porem mais giros gratis seriam brilhantes. Para encurtar, BR4Bet Casino e um clarao de emocoes para os viciados em emocoes de cassino! E mais o site e uma obra-prima de estilo radiante. dando vontade de voltar como uma chama eterna.
br4bet bonus|
joszaki regisztracio joszaki
накрутить подписчиков в тг
the lucky cat maneki neko oyunda uğur simvoludur. Daha ətraflı buradan baxın: [url=https://lucky-neko.com.az/]lucky neko slot[/url]. demo lucky neko test üçün gözəl imkandır.
slot demo lucky neko ilə məşq edə bilərsiniz. pg soft demo lucky neko sayəsində risksiz sınaq mümkündür. Buradan izləyin: [url=https://lucky-neko.com.az/]link lucky-neko.com.az[/url]. slot lucky neko demo əyləncə üçün əladır. slot demo lucky neko keyfiyyətli animasiyalara malikdir. slot lucky neko demo oynamaq çox əyləncəlidir. lucky neko slot demo sadə və rahatdır. lucky neko casino real pul üçün seçimdir.
lucky neko free play versiyası təcrübə üçün idealdır. Daha ətraflı buradan baxın: [url=https://lucky-neko.com.az/]lucky neko slot[/url]. pg soft demo lucky neko ilə risksiz oynamaq olar.
lucky cat lucky cat maneki neko kolleksiyası maraqlıdır. demo lucky neko pulsuz test üçündür. Bütün məlumat üçün: [url=https://lucky-neko.com.az/]lucky-neko.com.az[/url]. slot lucky neko demo əyləncə üçün əladır. slot demo lucky neko keyfiyyətli animasiyalara malikdir. maneki neko lucky cat qədim ənənələrdən gəlir. lucky cat maneki-neko uğur gətirən simvoldur. lucky neko gigablox slot əyləncəni artırır.
экскурсии в казанский кремль
как сделать много подписчиков в тг канале
suzgilliessmith – The combination of text and layouts makes me want to return often.
Sou louco pela energia de Brazino Casino, e uma mare de diversao que brilha como perolas. A colecao e uma onda de entretenimento. incluindo mesas com charme subaquatico. O suporte e uma concha de eficiencia. respondendo veloz como uma mare. Os pagamentos sao seguros e fluidos. porem mais giros gratis seriam uma loucura marinha. Na real, Brazino Casino garante um jogo que reluz como um coral para os exploradores de jogos online! Vale dizer a navegacao e facil como uma corrente marinha. dando vontade de voltar como uma onda.
brazino777.com/pt/|
Formula 1 canlı izləmə imkanı ilə yarışları qaçırmayın. Formula 1 canlı izləmə şifresiz seçimləri təqdim olunur.
Pilotlar haqqında geniş məlumat əldə edin ➡ [url=https://formula-1.com.az/]formula 1 drivers[/url].
Formula 1 izləmək üçün ən sərfəli onlayn xidmətlər. Formula 1 volunteer təcrübəsi ilə bağlı məsləhətlər.
Formula 1 2025 calendar üzrə bütün yarış tarixləri. Formula 1 izləmə zamanı texniki məlumatların üstünlükləri. Formula 1 movie izləyicilər üçün əyləncəli seçimdir. Formula 1 logo simvolikasının mənası barədə məlumat.
Je suis charme par Casinia Casino, resonne avec un rythme de casino chevaleresque. La selection du casino est une cour de plaisirs. proposant des slots de casino a theme medieval. Le service client du casino est un chevalier fidele. garantissant une assistance qui cadence. arrivent comme un tournoi. neanmoins des offres qui vibrent comme une cadence royale. Globalement, Casinia Casino enchante avec une rhapsodie de jeux pour les explorateurs de melodies en ligne! En plus offre un orchestre de couleurs medievales. ajoute une touche de grandeur au casino.
casinia casino abzocke|
Estou completamente ressonado por Stake Casino, tem uma energia de jogo tao pulsante quanto um eco em caverna. A selecao de titulos e um eco de emocoes. oferecendo sessoes ao vivo que ressoam como corais. O atendimento esta sempre ativo 24/7. oferecendo respostas claras como uma nota. As transacoes sao faceis como um sino. as vezes mais bonus regulares seriam harmonicos. No fim das contas, Stake Casino vale explorar esse cassino ja para quem curte apostar com estilo harmonico! Alem disso o design e fluido como uma onda sonora. adicionando um toque de eco ao cassino.
stake sauber|
Galera, vim dividir com voces sobre o Bingoemcasa porque achei muito alem do que esperava. O site tem um clima acolhedor que lembra um barzinho cheio de risadas. As salas de bingo sao com muita interacao, e ainda testei alguns caca-niqueis modernos, todos funcionaram redondinho. O atendimento no chat foi educado e prestativo, o que ja me deixou satisfeito. As retiradas foram instantaneas, inclusive testei PIX e caiu em minutos. Se pudesse apontar algo, diria que gostaria de ver mais brindes, mas nada que estrague a experiencia. Pra concluir, o Bingoemcasa me conquistou. Ja me sinto parte da comunidade
bingoemcasa .net|
Bakı Formula 1 trası ilə bağlı geniş təhlil təqdim olunur. Formula 1 canlı izləmə şifresiz seçimləri təqdim olunur.
Yarışlarla bağlı ətraflı məlumat üçün ➡ [url=https://formula-1.com.az/]http://formula-1.com.az[/url].
Formula 1 pilotları haqqında ətraflı bioqrafiyalar. Formula 1 Bakı 2024 biletləri onlayn əldə oluna bilər.
Formula 1 2025 calendar üzrə bütün yarış tarixləri. Formula 1 izləmə zamanı texniki məlumatların üstünlükləri. Formula 1 izləmə yolları haqqında faydalı təlimatlar. Formula 1 baku 2024 tickets artıq satışdadır.
Play slot games https://lemon-cazino-pl.com
Tennis betting https://betvisabengal.com
Cleaning is needed cleaning services toronto eco-friendly supplies, vetted cleaners, flat pricing, online booking, same-day options. Bonded & insured crews, flexible scheduling. Book in 60 seconds—no hidden fees.
Портал Чернівців https://58000.com.ua оперативні новини, анонси культурних, громадських та спортивних подій, репортажі з міста, інтерв’ю з чернівчанами та цікаві історії. Все про життя Чернівців — щодня, просто й доступно
Bahis dünyasının en güvenilir sitesine hoşgeldiniz. hemen giriş yapın deneme bonusunuzu alın!
Bakı Formula 1 trası ilə bağlı geniş təhlil təqdim olunur. Formula 1 üçün ən yaxşı bilet seçimlərini tapmaq mümkündür.
Məlumat üçün buraya klikləyin ➡ [url=https://formula-1.com.az/]formula 1 com az[/url].
Formula 1 pilotları haqqında ətraflı bioqrafiyalar. Formula 1 film və sənədli layihələrinə baxmaq mümkündür.
Formula 1 izləmək üçün ən yaxşı saytlar. Formula 1 izləmə zamanı texniki məlumatların üstünlükləri. Formula 1 canlı izləmə təcrübənizi paylaşın. Formula 1 car texnologiyası barədə maraqlı məqalələr.
Formula 1 canlı izləmə imkanı ilə yarışları qaçırmayın. Formula 1 sürücüləri haqqında maraqlı faktlar.
Ən son xəbərləri izləmək üçün baxın ➡ [url=https://formula-1.com.az/]formula 1[/url].
Formula 1 izləmək üçün ən sərfəli onlayn xidmətlər. Formula 1 Bakı 2024 biletləri onlayn əldə oluna bilər.
Formula 1 movie həvəskarları üçün tövsiyələr. Formula 1 volunteer olmaq istəyənlər üçün təcrübə. Formula 1 izləmə yolları haqqında faydalı təlimatlar. Formula 1 puan durumu ilə favorit komandanızı izləyin.
2024 və 2025 Formula 1 təqvimlərinin tam siyahısını əldə edin. Formula 1 logo və brendləşmə detallarını kəşf edin.
Simvol və brend haqqında oxuyun ➡ [url=https://formula-1.com.az/]formula 1 logo[/url].
Formula 1 canli yayım üçün yeni platformalar. Formula 1 Baku 2025 üçün ən maraqlı məlumatlar.
Formula 1 Baku track barədə dəqiq xəritə məlumatı. Formula 1 Azərbaycan mərhələsinin atmosferi barədə şərhlər. Formula 1 2024 takvimi ilə bağlı maraqlı dəyişikliklər. Formula 1 car texnologiyası barədə maraqlı məqalələr.
Acho simplesmente fenomenal DonaldBet Casino, e um cassino online que reluz como um picadeiro sob holofotes. A selecao de titulos e uma tenda de emocoes. com caca-niqueis modernos que encantam como acrobacias. O servico e confiavel como uma corda bamba. oferecendo respostas claras como um picadeiro. As transacoes sao simples como um malabarismo. em alguns momentos mais bonus regulares seriam circenses. Em resumo, DonaldBet Casino garante um jogo que reluz como um refletor para os apaixonados por slots modernos! Por sinal o site e uma obra-prima de estilo vibrante. elevando a imersao ao nivel de um espetaculo.
saque minimo donaldbet|
Curto demais o vortice de XPBet Casino, e um redemoinho de diversao que se repete. As opcoes sao ricas e giram como rodas. incluindo jogos de mesa com um toque eterno. O servico e confiavel como um ciclo. oferecendo respostas claras como uma roda. Os pagamentos sao seguros e fluidos. entretanto mais bonus regulares seriam ciclicos. Na real, XPBet Casino e um loop de emocoes para os viciados em emocoes de cassino! Vale dizer a navegacao e facil como um loop. adicionando um toque de loop ao cassino.
ultra bet xp|
Je suis envoute par Boomerang Casino, resonne avec un rythme de casino circulaire. Les options de jeu au casino sont riches et boomerang. offrant des lives qui pulsent comme un cercle. repond comme un boomerang parfait. offrant des solutions claires et instantanees. Le processus du casino est transparent et sans boucle. parfois des offres qui vibrent comme une cadence circulaire. A la fin, Boomerang Casino offre une experience de casino circulaire pour les amoureux des slots modernes de casino! Par ailleurs est une cadence visuelle envoutante. ajoute une touche de rythme arque au casino.
boomerang casino poker|
Bakı Formula 1 biletləri ilə bağlı ən sərfəli təklifləri araşdırın. Ən yaxşı Formula 1 filmləri və sənədli layihələri.
Yarışları canlı izləmək istəyənlər üçün keçid ➡ [url=https://formula-1.com.az/]formula 1 canlı izle[/url].
Formula 1 schedule daim yenilənir və asan izlənilir. Formula 1 volunteer təcrübəsi ilə bağlı məsləhətlər.
Formula 1 movie həvəskarları üçün tövsiyələr. Formula 1 haqqında ən çox verilən suallar. Formula 1 izləyiciləri üçün ən son xəbərlər. Formula 1 baku 2024 tickets artıq satışdadır.
Formula 1 puan durumu daim yenilənir və oyunçular üçün əlçatandır. Formula 1 üçün ən yaxşı bilet seçimlərini tapmaq mümkündür.
Ən yaxşı nəticələr və statistikalar üçün baxın ➡ [url=https://formula-1.com.az/]formula 1 standings[/url].
Formula 1 canlı izləmə ilə bağlı bütün təcrübələr. Formula 1 təqvimində dəyişikliklər barədə xəbərlər.
Formula 1 2025 calendar üzrə bütün yarış tarixləri. Formula 1 canlı yayım keyfiyyəti ilə bağlı rəylər. Formula 1 Bakı 2024 nə zaman keçiriləcək. Formula 1 drivers ilə bağlı ən son xəbərlər.
На этом сайте размещена интересная и полезная информация по многим вопросам.
Пользователи могут обнаружить ответы на популярные проблемы.
Материалы размещаются часто, чтобы каждый посетитель могли изучать свежую подборку.
Удобная организация сайта облегчает быстро найти нужные разделы.
анальное порно
Разнообразие тем делает ресурс универсальным для всех пользователей.
Каждый сможет подобрать сведения, которые подходят именно вам.
Существование доступных подсказок делает сайт ещё более значимым.
Таким образом, этот ресурс — это надёжный помощник полезной информации для каждого пользователей.
Смета на монтаж видеонаблюдения https://vcctv.ru
Мы работаем в торговле продуктами, и для нас маркировка имеет ключевое значение. После долгих сравнений остановились на оборудовании Labelaire, которое оказалось надёжным и удобным. Дополнительно приобрели принтеры для ценников и штрих-кодов, чтобы ускорить обновление ассортимента. Печать всегда чёткая, ярлыки держатся крепко. Система полностью оправдала ожидания, https://labelaire.ru/
Коллеги посоветовали мне читать Холд, когда я начал заниматься криптой. Должен сказать, что это один из лучших журналов по теме. В нём есть прогнозы, аналитика и даже обзоры инструментов для торговли. Особенно понравился раздел о кошельках — очень полезно для защиты активов https://thehold.ru/
інформаційний портал https://01001.com.ua Києва: актуальні новини, політика, культура, життя міста. Анонси подій, репортажі з вулиць, інтерв’ю з киянами, аналітика та гід по місту. Все, що треба знати про Київ — щодня, просто й цікаво.
інформаційний портал https://65000.com.ua Одеси та регіону: свіжі новини, культурні, громадські та спортивні події, репортажі з вулиць, інтерв’ю з одеситами. Всі важливі зміни та цікаві історії про життя міста — у зручному форматі щодня
Je suis accro a l’ambiance de Casombie, ca transporte dans un monde de chaos ludique. La gamme est un veritable apocalypse de fun, avec des slots au design effrayant. Le suivi est aussi fiable qu’un sortilege, repondant en un battement d’ailes. Les retraits sont rapides comme un mort-vivant en chasse, mais des tours gratuits supplementaires feraient frissonner. Au final, Casombie vaut chaque frisson qu’il procure pour ceux qui aiment l’adrenaline sombre ! A noter le design est sombre et captivant, ajoute une touche de magie noire.
casombie kasyno|
Je suis ensorcele par Freespin Casino, c’est une tornade de sensations vibrantes. Le catalogue est un kaleidoscope de delices, incluant des jeux de table d’une energie debordante. Les agents repondent plus vite qu’un eclair, offrant des solutions nettes comme un cristal. Les retraits sont rapides comme une fusee, mais des recompenses supplementaires seraient stellaires. En somme, Freespin Casino offre une experience aussi brillante qu’une comete pour les passionnes de cryptos ! En bonus la plateforme brille comme une constellation, facilite une experience fluide et radieuse.
free spin casino ndb codes|
Je suis booste par Robocat Casino, on detecte un blueprint de strategies innovantes. Le kit est un hub de diversite high-tech, incluant des blackjacks pour des reboots tactiques. Les techs interviennent avec une precision laser, garantissant une maintenance loyale dans le systeme. Les payouts dechargent via Bitcoin ou Ethereum, occasionnellement des add-ons de recompense additionnels scelleraient les connexions. En apotheose cybernetique, Robocat Casino construit un framework de divertissement automatise pour les guardians des reseaux numeriques ! En plus le graphisme est un render dynamique et immersif, incite a prolonger la session infinie.
robocat 270 mm fpv|
Je suis titille par MrPacho Casino, on discerne un jardin de defis appetissants. Les plats forment un tableau de textures innovantes, incluant des crash games comme JetX pour des pics de saveur. Le service officie en continu 24/7, avec une maestria qui anticipe les appetits. Les butins affluent via USDT ou fiat fluide, toutefois plus de hors-d’?uvre bonus journaliers agrementeraient le festin. En apotheose culinaire, MrPacho Casino convie a une exploration sans satiete pour les gardiens des buffets numeriques ! En sus le parcours est instinctif comme un arome familier, pousse a prolonger le banquet infini.
promotion mrpacho|
Je suis ebloui par Frumzi Casino, ca transporte dans un vortex de delices. La gamme est une deferlante de joies, proposant des paris sportifs qui font vibrer l’ame. Le suivi est d’une clarte etincelante, repondant en un battement de c?ur. Les paiements sont securises comme un recif, de temps a autre les offres pourraient etre plus explosives. Au final, Frumzi Casino merite d’etre explore sans attendre pour les joueurs en quete d’energie ludique ! En bonus le design est vibrant comme un recif corallien, amplifie l’immersion dans un ocean de fun.
frumzi deutschland|
Je suis incruste par RubySlots Casino, ca eleve le jeu a une coupe diamantaire. La collection de jeux est un ecrin debordant de plus de 200 joyaux exclusifs, integrant des progressifs comme Aztec’s Millions pour des mines de millions. Le support client est un lapidaire vigilant et constant, accessible par chat ou appel direct. Les gains jaillissent via Bitcoin ou cheques, toutefois des inclusions promotionnelles plus frequentes rehausseraient le portfolio. Dans l’ensemble de l’ecrin, RubySlots Casino se pose comme un pilier pour les joailliers pour les tailleurs des paris crypto ! En plus la circulation est instinctive comme une reflexion, simplifie la traversee des galeries ludiques.
promo codes for ruby slots|
Актуальное медоборудование играет ключевую функцию в обследовании и работе пациентов.
Больницы всё чаще используют высокотехнологичную технику.
Это даёт возможность врачам делать правильные заключения.
Новые приборы обеспечивают безопасность и для людей, и для медиков.
https://www.justmedia.ru/news/russiaandworld/apparaty-udarnovolnovoy-terapii-innovatsionnyy-podkhod-k-lecheniyu
Использование высоких технологий способствует качественное оздоровление.
Часто устройства содержат опции для точного мониторинга состояния здоровья.
Доктора могут оперативно принимать решения, основываясь на показателях аппаратуры.
Таким образом, современное медоборудование повышает уровень лечебного процесса.
Заботьтесь о здоровье сосудов ног с профессионалом! В группе «Заметки практикующего врача-флеболога» вы узнаете всё о профилактике варикоза, современных методиках лечения (склеротерапия, ЭВЛО), УЗИ вен и точной диагностике. Доверяйте опытному врачу — ваши ноги заслуживают лучшего: https://phlebology-blog.ru/
Je suis fleche par WildRobin Casino, ca eleve le jeu a une quete heroique. Il grouille d’une horde de quetes interactives, offrant des cashbacks et VIP 5 tiers des maitres comme Pragmatic Play et Evolution Gaming. L’assistance decoche des reponses precises, bandant des cordes multiples pour une frappe immediate. Les transferts sprintent stables et acceleres, occasionnellement les salves d’offres pourraient s’epaissir en densite. En decochant la derniere fleche, WildRobin Casino invite a une traque sans fin pour les archers de victoires astucieuses ! En fleche supplementaire la circulation est instinctive comme un tir, incite a prolonger la quete infinie.
robin schulz/cyril world gone wild|
Smart crypto trading https://terionbot.com with auto-following and DCA: bots, rebalancing, stop-losses, and take-profits. Portfolio tailored to your risk profile, backtesting, exchange APIs, and cold storage. Transparent analytics and notifications.
Soccer tətbiqi futbol həvəskarları üçün idealdır.
World soccer champs apk futbol həvəskarlarının seçimi olub. Futbol sevərlərə uyğun seçim [url=https://soccer.com.az/]www.soccer.com.az[/url] portalıdır.
Dream league soccer 2024 versiyası daha müasir imkanlar verir. Dream league soccer 2019 hələ də sevilən versiyalardandır. Soccer bros multiplayer futbol üçün əla seçimdir.
Soccer stream vasitəsilə canlı oyun izləmək mümkündür. Soccer field dizaynı oyunda çox real görsənir. Soccer super star futbolçular üçün maraqlıdır. World soccer champs hile son sürüm oyunçuların seçimi olub.
iOS üçün soccer yükləyib pulsuz oynayın.
Soccer 365 ilə futbol xəbərlərindən xəbərdar olun. Mobil oyunlar barədə öyrənmək üçün [url=https://soccer.com.az/]soccer com az[/url] linkinə keçid edin.
Bet365 soccer ilə idman mərclərinə qoşulun. Soccer star oyunçular üçün motivasiyaedici seçimdir. Dream league soccer 2020 yenilənmiş qrafiklər ilə fərqlənir.
Dream league soccer 2025 para və elmas hilesi ilə daha çox fürsət əldə edin. Soccer champs apk yükləmək çox asandır. Reddit soccer streams ilə matçlara çıxış tapmaq olar. Soccer champs para hilesi ilə oyunda üstünlük qazanın.
https://clickolov.ru/
Soccer oyununda komandalar seçmək çox asandır.
Soccer 365 ilə futbol xəbərlərindən xəbərdar olun. Yeni bacarıqlar qazanmaq istəyənlər [url=https://soccer.com.az/]soccer skills euro cup[/url] tətbiqini sınaya bilərlər.
Super liquid soccer fərqli futbol təcrübəsi bəxş edir. Soccer star oyunçular üçün motivasiyaedici seçimdir. Dream league soccer 2020 yenilənmiş qrafiklər ilə fərqlənir.
Soccer super star mod apk ilə ulduz olun. Dream league soccer 2016 köhnə versiya sevənlər üçün uyğundur. Sure soccer predictions mərc üçün yaxşı ipucları verir. World soccer champs hile son sürüm oyunçuların seçimi olub.
Je suis enflamme par Donbet Casino, il offre une aventure aussi intense qu’un volcan. Le catalogue est une explosion de diversite, proposant des paris sportifs qui font monter l’adrenaline. Le suivi est d’une clarte explosive, joignable a tout moment. Les transactions sont fiables et fluides, de temps a autre les offres pourraient etre plus volcaniques. Au final, Donbet Casino merite d’etre explore sans attendre pour les fans de casinos en ligne ! Cerise sur le gateau l’interface est fluide comme un torrent, donne envie de replonger dans la tempete.
donbet comment retirer son argent|
Je suis remue par Shuffle Casino, ca reinvente le jeu en une donne eternelle. Le deck est un etal de diversite aleatoire, incluant des roulettes pour des tours de table. Le support client est un croupier vigilant et incessant, accessible par bluff ou appel direct. Les retraits s’executent avec une souplesse remarquable, malgre cela des cartes de recompense additionnelles piocheaient les alliances. Pour clore la pioche, Shuffle Casino invite a une partie sans fin pour les croupiers des sabots numeriques ! En plus l’interface est un tapis navigable avec ruse, simplifie la traversee des paquets ludiques.
where is shuffle casino available|
Актуальное техническое оснащение играет ключевую функцию в обследовании и поддержке пациентов.
Медицинские центры всё чаще используют инновационную технику.
Это обеспечивает докторам делать быстрые заключения.
Современные приборы гарантируют безопасность и для больных, и для врачей.
https://seabeads.wordpress.com/over/?contact-form-id=1&contact-form-sent=224828&contact-form-hash=cb1bd203b52bea67b2570f70885a4859f9328829&_wpnonce=491bc39f2d
Внедрение высоких технологий ускоряет качественное оздоровление.
Часто устройства содержат опции для детального контроля состояния здоровья.
Специалисты могут быстро принимать решения, основываясь на данных аппаратуры.
Таким образом, новейшее медицинское оборудование улучшает уровень здравоохранения.
Je suis caramelise par Sugar Casino, ca transforme le jeu en une friandise eternelle. Le panier est une sucrerie de diversite petillante, integrant des lives comme Sweet Bonanza Candyland pour des eclats de sirop. Le service mijote en continu 24/7, assurant une attention fidele dans la patisserie. Le processus est lisse comme du caramel, a l’occasion des friandises de recompense additionnelles glaceraient les alliances. Pour clore le sirop, Sugar Casino devoile un plateau de triomphes fondants pour les gardiens des bonbonnieres numeriques ! De surcroit le graphisme est une praline dynamique et immersive, incite a prolonger la degustation infinie.
sugar pop 2 double dipped casino|
Компания СтройСинтез помогла нам построить большой дом из кирпича под ключ. Мы выбрали проект с тремя этажами и гаражом, и всё сделали точно в срок. Качество материалов и работ на высоте, дом получился надёжный и красивый. Спасибо за профессионализм: https://stroysyntez.com/
Soccer oyununda komandalar seçmək çox asandır.
Mini soccer star mod apk futbol oynamaq üçün əla seçimdir. Əlavə məlumat üçün [url=https://soccer.com.az/]http://soccer.com.az/[/url] səhifəsinə baxmaq olar.
Bet365 soccer ilə idman mərclərinə qoşulun. Pro soccer online multiplayer həvəskarları üçün uyğundur. Dream league soccer 2020 yenilənmiş qrafiklər ilə fərqlənir.
Soccer super star mod apk ilə ulduz olun. Soccer platform prediction nəticələri izləməyə kömək edir. Soccer live score ilə canlı nəticələri izləyin. World soccer champs hile son sürüm oyunçuların seçimi olub.
Сломалась машина? помощь авто на дороге мы создали профессиональную службу автопомощи, которая неустанно следит за безопасностью автомобилистов в Санкт-Петербурге и Ленинградской области. Наши специалисты всегда на страже вашего спокойствия. В случае любой нештатной ситуации — от банальной разрядки аккумулятора до серьёзных технических неисправностей — мы незамедлительно выезжаем на место.
Soccer oyununu telefonda oynamaq çox rahatdır.
Opera soccer tətbiqi ilə xəbərlərə tez çatın. Canlı matç izləmək istəyənlər üçün ən yaxşı seçim [url=https://soccer.com.az/]live soccer[/url] bölməsidir.
World soccer champs mod apk ilə əlavə imkanlar əldə edin. Rocket soccer derby fərqli futbol janrını təqdim edir. Real soccer 2012 nostalji sevənlər üçün uyğundur.
Dream league soccer 2025 para və elmas hilesi ilə daha çox fürsət əldə edin. Soccer champs apk yükləmək çox asandır. World soccer champs apk son sürüm daha yaxşı işləyir. World soccer champs apk 8.3.2 para hilesi ilə resurs artırın.
До SEO сайт не приносил заявок. Mihaylov Digital провели аудит, исправили ошибки и начали продвижение. Сейчас мы видим стабильный рост трафика и заявок. Отличное сотрудничество: https://mihaylov.digital/
Soccer oyununu telefonda oynamaq çox rahatdır.
Flashscore mobile livescore today soccer ilə ani nəticələr alın. Soccer haqqında daha ətraflı məlumat üçün [url=https://soccer.com.az/]soccer[/url] rəsmi səhifəsinə baxın.
Globe soccer turnirləri ilə dünya futboluna yaxın olun. Kit dream league soccer ilə komandanızı fərdiləşdirin. Soccer bros multiplayer futbol üçün əla seçimdir.
Dream league soccer 2025 para və elmas hilesi ilə daha çox fürsət əldə edin. World soccer champs maç atlama hilesi ilə oyun sürətlənir. Soccer super star futbolçular üçün maraqlıdır. Mini soccer star apk sadə və əyləncəlidir.
https://prykoly.ru/kak-pravilno-zakazat-servery-sistemy-hraneniya-dannyh-i-setevoe-oborudovanie-prakticheskoe-rukovodstvo/
Mobil soccer yükləyərək futbol əyləncəsini hər yerdə yaşayın.
Opera soccer tətbiqi ilə xəbərlərə tez çatın. Beynəlxalq turnirlər barədə ətraflı oxumaq üçün [url=https://soccer.com.az/]globe soccer[/url] bölməsi faydalıdır.
Soccer champs mod apk ilə oyun daha maraqlı olur. Kit dream league soccer ilə komandanızı fərdiləşdirin. World soccer champs apk para hilesi ilə əlavə resurs əldə edin.
World soccer champs hile apk oyunçular arasında məşhurdur. World soccer champs maç atlama hilesi ilə oyun sürətlənir. Pro evolution soccer 2019 hələ də çox oynanır. Soccer legends futbolun tarixi anlarını oyuna gətirir.
https://www.wildberries.ru/catalog/249860602/detail.aspx
Je suis titille par MrPacho Casino, on discerne un jardin de defis appetissants. Le menu est un cellier de variete exuberante, incluant des crash games comme JetX pour des pics de saveur. L’assistance distille des conseils affutes, avec une maestria qui anticipe les appetits. Les retraits glissent avec une souplesse remarquable, bien qu’ des amuse-bouches gratuits supplementaires rehausseraient les plats. En apotheose culinaire, MrPacho Casino tisse une tapisserie de divertissement gustatif pour les chasseurs de casinos virtuels ! En sus le portail est une salle a manger visuelle imprenable, pousse a prolonger le banquet infini.
pregled igralnice mrpacho|
Je suis irresistiblement couronne par SlotsPalace Casino, on percoit un cortege de man?uvres opulentes. Le royaume est un hall de diversite princiere, incluant des roulettes pour des tours de cour. Le service regne en continu 24/7, mobilisant des allegeances multiples pour une audience immediate. Le ceremonial est sculpte pour une fluidite imperiale, occasionnellement des sceaux de recompense additionnels forgeraient des dynasties. Pour clore le trone, SlotsPalace Casino sculpte une lignee de jeu grandiose pour les seigneurs des paris crypto ! De surcroit la structure rayonne comme un trone ancestral, amplifie l’immersion dans le royaume du jeu.
slots palace informations|
Je suis irresistiblement epice par PepperMill Casino, il concocte une alchimie de gains epoustouflants. La reserve de jeux est un herbier foisonnant de plus de 5 000 essences, avec des dice slots aux motifs piquants qui titillent les sens. Les artisans repondent avec une acuite remarquable, infusant des remedes limpides et immediats. Les flux coulent stables et acceleres, toutefois des elans promotionnels plus assidus animeraient le jardin. Dans la totalite du bouquet, PepperMill Casino convie a une exploration sans satiete pour les explorateurs de casinos virtuels ! En sus la trame irradie comme un elixir ancestral, intensifie l’envoutement dans le domaine des aromes.
458 peppermill rd kelso wa|
Je suis supercharge par Super Casino, on decele un escadron de strategies imparables. Le stock est un manuel de divertissements surpuissants, incluant des blackjacks pour des parades defensives. Le service patrouille en continu 24/7, assurant une protection fidele dans la zone. Les flux sont blindes par des boucliers crypto, toutefois des gadgets gratuits supplementaires rechargent les unites. Dans la globalite du QG, Super Casino construit un empire de divertissement invincible pour les pilotes des paris crypto ! Par surcroit la circulation est instinctive comme un jetpack, simplifie la traversee des zones ludiques.
super dragon casino game|
Мир гаджетов без воды https://indevices.ru честные обзоры, реальные замеры, фото/видео-примеры. Смартфоны, планшеты, аудио, гейминг, аксессуары. Сравнения моделей, советы по апгрейду, трекер цен и уведомления о скидках. Помогаем выбрать устройство под задачи.
Ремонт и стройка https://remontkit.ru без лишних затрат: инструкции, таблицы расхода, сравнение цен, контроль скрытых работ. База подрядчиков, отзывы, чек-листы, калькуляторы. Тренды дизайна, 3D-планировки, лайфхаки по хранению и зонированию. Практика и цифры.
Ваш портал о стройке https://gidfundament.ru и ремонте: материалы, инструменты, сметы и бюджеты. Готовые решения для кухни, ванной, спальни и террасы. Нормы, чертежи, контроль качества, приёмка работ. Подбор подрядчика, прайсы, акции и полезные образцы документов.
Все про ремонт https://lesnayaskazka74.ru и строительство: от идеи до сдачи. Пошаговые гайды, электрика и инженерия, отделка, фасады и кровля. Подбор подрядчиков, сметы, шаблоны актов и договоров. Дизайн-инспирации, палитры, мебель и свет.
Все про ремонт https://lesnayaskazka74.ru и строительство: от идеи до сдачи. Пошаговые гайды, электрика и инженерия, отделка, фасады и кровля. Подбор подрядчиков, сметы, шаблоны актов и договоров. Дизайн-инспирации, палитры, мебель и свет.
Ремонт и строительство https://nastil69.ru от А до Я: планирование, закупка, логистика, контроль и приёмка. Калькуляторы смет, типовые договора, инструкции по инженерным сетям. Каталог подрядчиков, отзывы, фото-примеры и советы по снижению бюджета проекта.
Нужен аккумулятор? dostavka-akb-spb в наличии: топ-бренды, все размеры, правый/левый токовывод. Бесплатная проверка генератора при установке, trade-in старого АКБ. Гарантия до 3 лет, честные цены, быстрый самовывоз и курьер. Поможем выбрать за 3 минуты.
Хочешь сдать акб? сдать старый аккумулятор честная цена за кг, моментальная выплата, официальная утилизация. Самовывоз от 1 шт. или приём на пункте, акт/квитанция. Безопасно и законно. Узнайте текущий тариф и ближайший адрес.
Дети Ветра Кайт – это специально разработанный воздушный змей, используемый для занятий кайтсёрфингом, сноукайтингом, лендкайтингом и другими видами спорта, где используется тяга ветра. Он состоит из купола (ткани), строп (линий управления) и планки (ручки управления), позволяющих райдеру контролировать его движение и тягу. Размер кайта выбирается в зависимости от силы ветра и веса райдера.
Ищешь аккумулятор? аккумуляторы автомобильные купить в спб AKB SHOP занимает лидирующие позиции среди интернет-магазинов автомобильных аккумуляторов в Санкт-Петербурге. Наш ассортимент охватывает все категории транспортных средств. Независимо от того, ищете ли вы надёжный аккумулятор для легкового автомобиля, мощного грузовика, комфортного катера, компактного скутера, современного погрузчика или специализированного штабелёра
Нужен надежный акб? акб купить в спб AKB STORE — ведущий интернет-магазин автомобильных аккумуляторов в Санкт-Петербурге! Мы специализируемся на продаже качественных аккумуляторных батарей для самой разнообразной техники. В нашем каталоге вы найдёте идеальные решения для любого транспортного средства: будь то легковой или грузовой автомобиль, катер или лодка, скутер или мопед, погрузчик или штабелер.
Ich bin vollig hin und weg von King Billy Casino, es verstromt eine Spielstimmung, die wie ein Palast glanzt. Die Casino-Optionen sind vielfaltig und prachtig, mit einzigartigen Casino-Slotmaschinen. Die Casino-Mitarbeiter sind schnell wie ein koniglicher Bote, ist per Chat oder E-Mail erreichbar. Casino-Transaktionen sind simpel wie ein koniglicher Befehl, manchmal mehr Casino-Belohnungen waren ein furstlicher Gewinn. Am Ende ist King Billy Casino ein Casino, das man nicht verpassen darf fur die, die mit Stil im Casino wetten! Zusatzlich die Casino-Plattform hat einen Look, der wie ein Kronungsmantel glanzt, das Casino-Erlebnis total veredelt.
king billy casino canada|
Je suis fou de Celsius Casino, ca pulse avec une energie de casino ardente. Les choix de jeux au casino sont varies et enflammes, comprenant des jeux de casino optimises pour les cryptomonnaies. Le service client du casino est une torche eclatante, repondant en un eclair ardent. Les retraits au casino sont rapides comme une braise, cependant plus de tours gratuits au casino ce serait enflamme. Globalement, Celsius Casino est une pepite pour les fans de casino pour les passionnes de casinos en ligne ! Par ailleurs le site du casino est une merveille graphique ardente, amplifie l’immersion totale dans le casino.
celsius casino no deposit bonus codes|
J’adore a fond 7BitCasino, il offre une sensation de casino unique. La selection de jeux est colossale, incluant des slots de pointe. Le personnel offre un accompagnement irreprochable, joignable a toute heure. Les retraits sont ultra-rapides, par moments les promotions pourraient etre plus genereuses, ou des tournois avec des prix plus eleves. Globalement, 7BitCasino est un incontournable pour les adeptes de sensations fortes ! En bonus l’interface est fluide et retro, facilite chaque session de jeu.
bitstarz casino vs 7bitcasino|
Сделать мед лицензию стало удобно благодаря профессиональной поддержке центра: сотрудники подготовили пакет документов, проверили его на соответствие требованиям, сопровождали подачу и консультировали по всем вопросам https://licenz.pro/
В Кухни в Дом мы купили кухню на заказ с уникальным дизайном. Проект получился стильным, а результат — просто великолепным. Отличная компания, которой можно доверять – https://kuhni-v-dom.ru/
Lucky Mate is an online casino for Australian players, offering pokies, table games, and live dealer options. It provides a welcome bonus up to AUD 1,000, accepts Visa, PayID, and crypto with AUD 20 minimum deposit, and has withdrawal limits of AUD 5,000 weekly. Licensed, it promotes safe play: Lucky Mate Casino
Je suis totalement conquis par Betzino Casino, c’est une veritable aventure pleine de frissons. Il y a une profusion de jeux varies, offrant des sessions de casino en direct immersives par Evolution Gaming. Le service client est exceptionnel, joignable par email ou chat. Les retraits sont rapides, souvent traites en 24 heures pour les e-wallets, bien que plus de tours gratuits seraient un atout. Dans l’ensemble, Betzino Casino est un incontournable pour les joueurs en quete d’adrenaline ! Ajoutons que le site est concu avec elegance et ergonomie, ce qui amplifie le plaisir de jouer.
betzino abzocke|
Получение лицензии на медицинскую деятельность с Журавлев Консалтинг Групп прошло гладко, специалисты подготовили документы и сопровождали процесс подачи до официального разрешения: https://licenz.pro/
Обратились в Окна в СПб для замены старых окон в квартире. Сотрудники приехали вовремя, сделали точные замеры и предложили хорошие варианты. Установку выполнили аккуратно и профессионально. Мы остались очень довольны результатом: https://okna-v-spb.ru/
This site offers tons of interesting and helpful information.
On this platform, you can explore a wide range of topics that cover many important themes.
Every post is prepared with care to clarity.
The content is regularly updated to keep it relevant.
Visitors can get something new every time they browse.
It’s an excellent place for those who appreciate educational reading.
A lot of visitors consider this website to be reliable.
If you’re looking for well-written information, you’ll definitely discover it here.
https://arnona.biz
LaLiga canlı izləmə üçün ideal mənbədir. Ən çox xal toplayan komandanı buradan öyrən.
Canlı LaLiga matçlarını izləmək üçün ən rahat platforma. LaLiga logo png fayllarını tapmaq asandır.
Hər turdan sonra xal durumu yenilənir — [url=https://laliga.com.az/]LaLiga puan durumu[/url] səhifəsini izləyin.
Ən çox qol vuran LaLiga futbolçuları kimlərdir?.
LaLiga fixtures 2022/23 və 2025 siyahısı.
LaLiga statistika səhifəsində komanda göstəriciləri.
LaLiga champions list artıq dərc olunub.
Futbol azarkeşləri üçün LaLiga livescore bölməsi.
Je suis accro a Instant Casino, il propose une experience de casino explosive. Les options de jeu en casino sont ultra-riches, comprenant des jeux de casino tailles pour les cryptos. Le support du casino est dispo 24/7, avec une aide qui pete le feu. Les gains du casino arrivent a la vitesse lumiere, mais bon des recompenses de casino en plus ca ferait kiffer. Au final, Instant Casino garantit un fun de casino supersonique pour les fans de casinos en ligne ! A noter aussi le site du casino est une tuerie graphique, donne envie de replonger dans le casino direct.
club dice instant play casino|
Hər turun nəticələri real vaxtda yenilənir. LaLiga turnir cədvəlində mövqeləri izləmək maraqlıdır.
Futbol sevərlərə LaLiga canlı izləmə təcrübəsi. LaLiga logo 2020 ilə 2025 arasındakı fərqləri gör.
Matç cədvəlini izləmək üçün sadəcə [url=https://laliga.com.az/]laliga.com.az[/url] saytına daxil olun və təqvimi yoxlayın.
Bellingham LaLiga-da tarixi nəticələr göstərir.
LaLiga turnir jadvali yeniləndi.
LaLiga teams haqqında maraqlı faktlar.
LaLiga champions 2023 komandaları arasında böyük rəqabət.
LaLiga nəticələri ilə favoritlərinizi izləyin.
Ich bin suchtig nach DrueGlueck Casino, es liefert einen einzigartigen Adrenalinkick. Es gibt eine Flut an abwechslungsreichen Casino-Titeln, mit einzigartigen Casino-Slotmaschinen. Der Casino-Service ist mega zuverlassig, antwortet in Sekundenschnelle. Auszahlungen im Casino sind blitzschnell, trotzdem mehr Freispiele im Casino waren top. Alles in allem ist DrueGlueck Casino ein Casino, das man nicht verpassen darf fur die, die mit Stil im Casino wetten! Ubrigens die Casino-Navigation ist kinderleicht, den Spielspa? im Casino maximiert.
drueckglueck mobile|
Je trouve absolument epoustouflant 1xbet Casino, ca procure une plongee dans un univers palpitant. Les options de jeu sont riches et diversifiees, proposant des jeux de table elegants et classiques. Le service client est exceptionnel, avec un suivi de qualite. Les gains sont verses en un temps record, neanmoins les promotions pourraient etre plus genereuses. Dans l’ensemble, 1xbet Casino ne decoit jamais pour ceux qui aiment parier ! Notons egalement que la navigation est intuitive et rapide, renforce l’immersion totale.
1xbet free download|
İspaniya çempionatının ən maraqlı matçlarını buradan izləyin. LaLiga standings səhifəsi ilə hər şeyi bil.
Canlı LaLiga matçlarını izləmək üçün ən rahat platforma. LaLiga logo 2020 ilə 2025 arasındakı fərqləri gör.
Matçların canlı nəticələrini [url=https://laliga.com.az/]LaLiga live[/url] vasitəsilə izləmək mümkündür.
LaLiga goal record yenidən dəyişdi.
LaLiga fixtures 2022/23 və 2025 siyahısı.
Komandaların performans statistikasını müqayisə et.
Ən çox titul qazanan LaLiga klubları hansılardır?.
LaLiga nəticələri ilə favoritlərinizi izləyin.
İspaniya futbolunun ritmini hiss et. LaLiga cədvəlini izləmək istəyirsinizsə, ən son məlumatlar buradadır.
LaLiga canlı nəticələri anında görün. LaLiga logo 2020 ilə 2025 arasındakı fərqləri gör.
Hər turdan sonra xal durumu yenilənir — [url=https://laliga.com.az/]LaLiga puan durumu[/url] səhifəsini izləyin.
Ən çox LaLiga qazanan komanda hansıdır?.
LaLiga maçlar və canlı yayım üçün bələdçi.
LaLiga teams haqqında maraqlı faktlar.
LaLiga champions list artıq dərc olunub.
LaLiga scoreboard dərhal yenilənir.
İspaniya futbolunun ritmini hiss et. İspaniyanın lider komandalarını tanı.
İspaniya LaLiga canlı futbolunu qaçırmayın. Futbol dizayn sevərləri üçün LaLiga logo nümunələri.
Yeni “LaLiga EA Sports” loqosu və turnir xəbərləri üçün [url=https://laliga.com.az/]laliga ispaniya[/url] səhifəsinə baxın.
Ən çox LaLiga qazanan komanda hansıdır?.
LaLiga matçlar hansı kanalda yayımlanır?.
LaLiga statistika səhifəsində komanda göstəriciləri.
LaLiga kupası uğrunda mübarizə həyəcanı.
Spaniyada futbolun nəbzi LaLiga ilə vurur.
LaLiga oyunlarını izləmək indi çox asandır. LaLiga turnir cədvəlində mövqeləri izləmək maraqlıdır.
LaLiga tv live yayımını izləmək üçün link buradadır. LaLiga rəsmi loqosu artıq yeni rənglərlə.
Futbol dünyasından ən son xəbərlər [url=https://laliga.com.az/]laliga.com.az[/url] ünvanında. Canlı izləmə və təhlillər üçün mükəmməl platformadır.
Hər mövsüm LaLiga top scorers fərqlənir.
LaLiga turnir jadvali yeniləndi.
LaLiga defenders və midfielders siyahısı.
İspaniya LaLiga kupası hər il diqqət çəkir.
LaLiga oyunlarının qrafiki və nəticələri burada.
Складчик — это сервис, которому можно доверять. Я участвовал уже в нескольких складчинах и всегда получал материалы вовремя. Качество отличное, цены минимальные. Очень доволен: https://v27.skladchik.org/
https://ozon.ru/t/csgBrx8
LaLiga statistikası hər gün yenilənir. Yeni mövsüm üçün LaLiga sıralaması artıq dərc olunub.
Bu həftə LaLiga canlı oyunları çox maraqlıdır. Loqodakı dəyişikliklər brendin müasir ruhunu əks etdirir.
Futbol xəbərlərini izləmək üçün bu linki yoxlayın — [url=https://laliga.com.az/]www.laliga.com.az[/url].
LaLiga goal of the season 2022 hələ də yadda qalır.
Bu həftənin LaLiga oyunları çox həyəcanlı olacaq.
LaLiga takımları arasında rəqabət yüksəkdir.
Ən çox titul qazanan LaLiga klubları hansılardır?.
İspaniya LaLiga futbol atmosferini yaşayın.
Актуальное медицинское оборудование играет ключевую часть в обследовании и поддержке пациентов.
Медицинские центры всё чаще оборудуются передовую системы.
Это обеспечивает докторам проводить быстрые диагнозы.
Новые приборы обеспечивают комфорт и для пациентов, и для персонала.
http://liecebnarieka.sk/forums/topic/blazerifdkf449-4/page/138/#post-3499379
Развитие высоких технологий ускоряет эффективное лечение.
Большинство устройства включают функции для детального контроля состояния здоровья.
Специалисты могут своевременно реагировать, основываясь на данных аппаратуры.
Таким образом, новейшее медоборудование повышает качество медицины.
I’m gone to inform my little brother, that he should also pay a quick visit this website on regular basis to take updated from hottest gossip.
keepstyle
Актуальные новости автопрома https://myrexton.ru свежие обзоры, тест-драйвы, новые модели, технологии и тенденции мирового автомобильного рынка. Всё самое важное — в одном месте.
Строительный портал https://stroimsami.online новости, инструкции, идеи и лайфхаки. Всё о строительстве домов, ремонте квартир и выборе качественных материалов.
Новостной портал https://daily-inform.ru с последними событиями дня. Политика, спорт, экономика, наука, технологии — всё, что важно знать прямо сейчас.
J’adore le delire total de Gamdom, on dirait une explosion de fun. Il y a un tsunami de titres varies, avec des slots qui claquent grave. Le service client est une tuerie, avec une aide qui dechire tout. Les retraits sont rapides comme un ninja, mais bon des bonus plus reguliers ce serait la classe. Pour resumer, Gamdom est un spot a ne pas louper pour les accros aux sensations extremes ! Cote plus le site est une tuerie graphique, booste l’immersion a fond les ballons.
how much is one gamdom coin|
Je suis totalement envoute par FatPirate, c’est une plateforme qui envoie du lourd. Le choix de jeux est monumental, avec des slots qui dechirent. Les agents sont rapides comme l’eclair, avec une aide qui dechire. Les transactions sont simples comme bonjour, par moments les offres pourraient etre plus genereuses. Au final, FatPirate offre une experience de ouf pour les pirates des slots modernes ! Et puis le site est une pepite visuelle, ajoute un max de swag.
fatpirate casino no deposit bonus|
Online bahis sitesi Vegabet’e giriş yapmak çok kolay. Hemen Vegabet’e üye ol bahiste kazanmaya başla. https://www.vegabets.net/
Tightrope Game – a balance challenge with obstacles. Quick, addictive, and perfect for testing focus: Tightrope game rules and strategies
Ich bin total fasziniert von Snatch Casino, es ist eine dynamische Erfahrung. Es gibt eine unglaubliche Vielfalt an Spielen, mit modernen und fesselnden Slots. Der Support ist 24/7 verfugbar, bietet prazise Losungen. Die Zahlungen sind flussig und sicher, trotzdem mehr variierte Boni waren toll. Insgesamt Snatch Casino ist ein Must fur Spieler fur Casino-Fans ! Daruber hinaus die Site ist stylish und schnell, was das Spielvergnugen steigert.
snatch casino app|
Estou seduzido por Flabet Casino, parece um turbilhao de prazer. O catalogo e simplesmente gigantesco, compreendendo milhares de jogos adaptados para criptos. O servico e de uma eficiencia notavel, contatavel a qualquer momento. O processo e simples e sem problemas, no entanto gostaria de mais bonus variados. Em resumo, Flabet Casino garante uma experiencia de jogo top para os amantes de adrenalina ! De mais a mais a plataforma e visualmente top, torna cada sessao imersiva.
flabet papa|
Je trouve completement fou Instant Casino, c’est un casino en ligne qui envoie du lourd. La selection de titres de casino est dingue, comprenant des jeux de casino tailles pour les cryptos. Le crew du casino assure un suivi de ouf, joignable par chat ou email. Les transactions de casino sont simples comme un neon, des fois des bonus de casino plus reguliers ce serait top. Bref, Instant Casino est un casino en ligne qui cartonne pour les pirates des slots de casino modernes ! Cote plus le site du casino est une tuerie graphique, facilite le delire total au casino.
kudos casino instant play|
A vibrant platformer guiding a panda through exciting puzzles and treasures. Fun and family-friendly: new online games from Canada
Je suis pactise avec Mafia Casino, c’est un empire ou chaque pari scelle un accord de fortune. Il pullule d’une legion de complots interactifs, avec des slots aux themes gangster qui font chanter les rouleaux. Le service conspire en continu 24/7, avec une ruse qui anticipe les traitrises. Les transferts glissent stables et acceleres, occasionnellement des largesses gratuites supplementaires boosteraient les operations. En scellant le pacte, Mafia Casino construit un syndicate de divertissement impitoyable pour les parrains de casinos virtuels ! De surcroit le portail est une planque visuelle imprenable, incite a prolonger l’intrigue infinie.
mafia casino test|
Ich finde es unglaublich Snatch Casino, es ist eine dynamische Erfahrung. Die Optionen sind umfangreich und abwechslungsreich, mit modernen und fesselnden Slots. Die Hilfe ist schnell und professionell, erreichbar jederzeit. Die Zahlungen sind flussig und sicher, obwohl mehr variierte Boni waren toll. Zusammenfassend Snatch Casino ist eine au?ergewohnliche Plattform fur Adrenalin-Junkies ! Daruber hinaus das Design ist ansprechend und intuitiv, erleichtert die Gesamterfahrung.
snatch casino test|
Мен ар дайым Лука Модричти көргөндө чыныгы лидерликти байкайм.Модричсиз Реалды элестетүү кыйын. Модрич — чыныгы футбол интеллекти. Анын жашы эмес, оюну аны аныктайт. Колдон бетер esli kerek болсо, бул форматты да эстеп кал: [url=https://luka-modric-kg.com/]luka-modric-kg com[/url]. Лука Модрич ЧМ 2022де дагы мыкты оюн көрсөттү.
Футбол сүйүүчүлөрү үчүн Модрич — Реалдын жүрөгү. Бул адамдын жашоосу — чыныгы күрөш. Анын баасы трансфермаркетте дагы жогору. Футбол дүйнөсү Модричсиз элестетүү кыйын.
Сео аудит сайта онлайн https://seo-audit-sajta.ru
Need TRON Energy? rent tron energy instantly and save on TRX transaction fees. Rent TRON Energy quickly, securely, and affordably using USDT, TRX, or smart contract transactions. No hidden fees—maximize the efficiency of your blockchain.
Je suis integre a Mafia Casino, ca forge un syndicate de defis impitoyables. La reserve est un code de divertissements mafieux, proposant des crash pour des chutes de pouvoir. Le service conspire en continu 24/7, avec une ruse qui anticipe les traitrises. Les flux sont masques par des voiles crypto, occasionnellement des complots promotionnels plus frequents dynamiseraient le territoire. A la fin de cette conspiration, Mafia Casino construit un syndicate de divertissement impitoyable pour les conspirateurs de victoires rusees ! Par surcroit la circulation est instinctive comme un chuchotement, ce qui propulse chaque pari a un niveau de don.
mafia casino download for android|
Лука Модрич футбол дүйнөсүнүн символу болуп калды.Лука Модрич Мадрид үчүн тарых жараткан. Модрич — чыныгы футбол интеллекти. Жаш өткөн сайын Модричтин тажрыйбасы баалуу болуп жатат. Колдон бетер esli kerek болсо, бул форматты да эстеп кал: [url=https://luka-modric-kg.com/]luka-modric-kg com[/url]. Лука Модрич Хорватия үчүн сыймык.
Лука Модрич — Реалдын акыл-эс борбору. Бул адамдын жашоосу — чыныгы күрөш. Бул оюнчу ар бир клуб үчүн кымбат сыйлык болот. Модрич карьерасын кайда улантса да, күйөрмандар аны колдойт.
Creative photography often focuses on revealing the beauty of the body lines.
It is about light rather than appearance.
Skilled photographers use soft lighting to convey atmosphere.
Such images emphasize delicacy and individuality.
https://xnudes.ai/
Every frame aims to show emotion through pose.
The intention is to show natural harmony in an elegant way.
Observers often value such work for its emotional power.
This style of photography unites emotion and sensitivity into something truly expressive.
Футболдо мындай оюнчу сейрек кездешет, Модрич өзгөчө.Модричсиз Реалды элестетүү кыйын. Футболдо мындай туруктуу статистика аз кездешет. Көптөр “сколько лет Лука Модричке?” деп сурашат — бирок ал үчүн жаш маанилүү эмес. Легенданын бардык трофейлери бир тизмекте — ыңгайлуу окула турган: [url=https://luka-modric-kg.com/]Лука Модрич трофеи[/url]. Хорват футболунун жандуу легендасы.
Анын Реалдагы карьерасы алтын барактар менен жазылган. Бул адам чынында эле чыныгы чемпион. Модрич акчадан мурда сыймыкты ойлойт. Анын тажрыйбасы каалаган клубга керектүү.
Ich kann nicht genug bekommen von NV Casino, es erzeugt eine Energie, die suchtig macht. Die Vielfalt der Titel ist atemberaubend, mit Spielen, die perfekt fur Kryptos geeignet sind. Die Hilfe ist effizient und professionell, bietet klare Antworten. Die Gewinne kommen ohne Verzogerung, ab und zu zusatzliche Freispiele waren toll. Insgesamt, NV Casino ist eine Plattform, die rockt fur Spieler auf der Suche nach Action ! Au?erdem die Oberflache ist intuitiv und stylish, macht die Erfahrung flussiger.
https://playnvcasino.de/|
Need porn videos or photos? generate ai porn images – create erotic content based on text descriptions. Generate porn images, videos, and animations online using artificial intelligence.
IPTV форум vip-tv.org.ua место, где обсуждают интернет-телевидение, делятся рабочими плейлистами, решают проблемы с плеерами и выбирают лучшие IPTV-сервисы. Присоединяйтесь к сообществу интернет-ТВ!
Футболдо мындай оюнчу сейрек кездешет, Модрич өзгөчө.Бул футбол дүйнөсүндө чоң өзгөрүү болот. Модричтин пас тактыгы укмуштуудай. Модричтин энергиясы таң калтырат. Объективдүү маалымат жана факт-чек менен таанышыңыз: [url=https://luka-modric-kg.com/]luka-modric-kg.com[/url]. Лука Модрич дүйнөлүк футболго башкача дем берди.
Лука Модрич — Реалдын акыл-эс борбору. Модрич жаш кезинен эле футболго берилген. Көптөр “Модрич канча табат?” деп сурашат. Модрич карьерасын кайда улантса да, күйөрмандар аны колдойт.
Модрич сыяктуу туруктуу оюн көрсөтүү абдан кыйын.Лука Модрич Реалдын жүрөгү болгон. Лука Модрич статистика дайыма жогорку деңгээлде. Жаш өткөн сайын Модричтин тажрыйбасы баалуу болуп жатат. Реалдагы доору, ЛЧ финалдары жана рекорддор — баары ушул жакта: [url=https://luka-modric-kg.com/]https://luka-modric-kg.com/[/url]. Анын чеберчилиги ар бир эл аралык турнирде көрүнөт.
Модрич Реалдын орто талаасын башкарып келет көп жылдан бери. Модрич согуш жылдарында чоңойгон, бирок футбол аны сактап калды. Анын эмгеги ар тыйынга татыктуу. Эми ал Миланга өтөт деген ушак бар.
Acho incrivel Flabet Casino, e uma experiencia vibrante. A gama de jogos e impressionante, compreendendo milhares de jogos adaptados para criptos. O servico ao cliente e top, oferecendo solucoes precisas. O processo e simples e sem problemas, as vezes mais rodadas gratis seriam um plus. Em conclusao, Flabet Casino oferece prazer garantido para os fas de apostas online ! De mais a mais o design e atraente e intuitivo, reforca o desejo de voltar.
flabet camisa flamengo|
Ich bin total fasziniert von Snatch Casino, es ist eine dynamische Erfahrung. Die Optionen sind umfangreich und abwechslungsreich, mit immersiven Live-Sitzungen. Die Hilfe ist schnell und professionell, erreichbar jederzeit. Die Gewinne kommen schnell, gelegentlich die Angebote konnten gro?zugiger sein. Global Snatch Casino lohnenswert fur Spieler auf der Suche nach Spa? ! Au?erdem die Oberflache ist flussig und modern, verstarkt den Wunsch zuruckzukehren.
snatch casino 50 fs|
Мен ар дайым Лука Модричти көргөндө чыныгы лидерликти байкайм.Лука Модрич Реалдын жүрөгү болгон. Модрич — чыныгы футбол интеллекти. Жаш өткөн сайын Модричтин тажрыйбасы баалуу болуп жатат. Статс жана графикалар менен ыңгайлуу бөлүм бар — карап көрүңүз: [url=https://luka-modric-kg.com/]Лука Модрич stats[/url]. Лука Модрич дүйнөлүк футболго башкача дем берди.
Модрич менен Кроос — эң мыкты дуэттердин бири. Анын автобиографиясы мотивация берет. Футбол дүйнөсүндө андай адептүү оюнчулар аз. Модрич карьерасын кайда улантса да, күйөрмандар аны колдойт.
Футболдо мындай оюнчу сейрек кездешет, Модрич өзгөчө.Көпчүлүк күйөрмандар Модричтин кетишине ишенбей жатат. Футболдо мындай туруктуу статистика аз кездешет. Лука Модричтин жашына ишенүү кыйын, ал дагы деле чоку формасында. Ийгиликтер тарыхын окуп шыктанып ал: [url=https://luka-modric-kg.com/]Лука Модрич биография[/url]. Модричсиз Хорватияны элестетүү мүмкүн эмес.
Модрич менен Кроос — эң мыкты дуэттердин бири. Бул адам чынында эле чыныгы чемпион. Лука Модричтин айлык маянасы укмуш. Футбол дүйнөсү Модричсиз элестетүү кыйын.
Je suis pactise avec Mafia Casino, c’est un empire ou chaque pari scelle un accord de fortune. La cache de jeux est un arsenal cache de plus de 5000 armes, incluant des roulettes pour des tours de table. Le service conspire en continu 24/7, avec une ruse qui anticipe les traitrises. Les retraits s’executent avec une furtivite remarquable, malgre cela des largesses gratuites supplementaires boosteraient les operations. En scellant le pacte, Mafia Casino devoile un plan de triomphes secrets pour ceux qui ourdissent leur destin en ligne ! De surcroit la structure vibre comme un code ancestral, ce qui propulse chaque pari a un niveau de don.
mafia casino promo code no deposit bonus|
Современные технологии 3D-печати — это инновационный инструмент, который даёт возможность быстро изготавливать прототипы и готовые изделия. Мы оказываем услуги 3D-печати по доступным ценам. Наша команда подбираем оптимальные материалы, что гарантирует высокую точность результата. Применяются современные и прочные составы, благодаря чему печать подойдёт для архитектуры, прототипирования и хобби. Сроки выполнения заказа минимальны, а мы предлагаем выгодные условия. Перейдите по ссылке, чтобы заказать, и мы напечатаем всё, что вам необходимо https://r12imob.store/index.php?page=user&action=pub_profile&id=621595. Мы обеспечиваем надежность и долговечность всех изделий.
Всё о металлообработке j-metall.ru/ и металлах: технологии, оборудование, сплавы и производство. Советы экспертов, статьи и новости отрасли для инженеров и производителей.
Ich bin fasziniert von SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Der Katalog ist reichhaltig und variiert, mit Spielen, die fur Kryptos optimiert sind. Der Kundenservice ist ausgezeichnet, bietet klare Losungen. Die Zahlungen sind sicher und smooth, trotzdem mehr Rewards waren ein Plus. Zusammengefasst, SpinBetter Casino bietet unvergessliche Momente fur Adrenalin-Sucher ! Au?erdem die Navigation ist kinderleicht, was jede Session noch besser macht. Hervorzuheben ist die schnellen Einzahlungen, die Flexibilitat bieten.
spinbettercasino.de|
Ich liebe die Atmosphare von NV Casino, es erzeugt eine Energie, die suchtig macht. Die Vielfalt der Titel ist atemberaubend, mit Slots im innovativen Design. Die Hilfe ist effizient und professionell, mit praziser Unterstutzung. Die Zahlungen sind sicher und flussig, dennoch mehr abwechslungsreiche Boni waren willkommen. Zum Abschluss, NV Casino ist definitiv empfehlenswert fur Krypto-Liebhaber ! Daruber hinaus die Oberflache ist intuitiv und stylish, was jede Session noch spannender macht.
https://playnvcasino.de/|
https://kiteschoolhurghada.ru/
Есть металлолом? сдать металлолом в санкт-петербурге мы предлагаем полный цикл услуг по приему металлолома в Санкт-Петербурге, включая оперативную транспортировку материалов непосредственно на перерабатывающий завод. Особое внимание мы уделяем удобству наших клиентов. Процесс сдачи металлолома организован максимально комфортно: осуществляем вывоз любых объемов металлических отходов прямо с вашей территории.
Хочешь сдать металл? 112lomvspb наша компания специализируется на профессиональном приёме металлолома уже на протяжении многих лет. За это время мы отточили процесс работы до совершенства и готовы предложить вам действительно выгодные условия сотрудничества. Мы принимаем практически любые металлические изделия: от небольших профилей до крупных металлоконструкций.
Hey there I am so excited I found your website, I really found you by accident, while I was researching on Digg for something else, Regardless I am here now and would just like to say thanks for a marvelous post and a all round entertaining blog (I also love the theme/design), I don’t have time to read it all at the moment but I have saved it and also added in your RSS feeds, so when I have time I will be back to read a lot more, Please do keep up the awesome jo.
https://silvestry.com.ua/iak-pryskoryty-protses-rozboru-far-za-dopomohoiu-pichky.html
официальный сайт ПокерОК
ПокерОК
I’m amazed by Wazamba Casino, it’s an adventure that throbs with excitement. The catalog is absolutely massive, featuring over 5,000 titles from top providers. Plus 200 free spins to start strong. Agents respond swiftly. Withdrawals are processed quickly, though generous offers could be expanded. All in all, Wazamba Casino is essential for gamers for casino enthusiasts ! In addition the site is fast and responsive, simplifies the overall experience. Another perk is the loyalty program with masks, fostering a community feel.
https://wazambagr.com/|
вешалка недорого
Алгачкы жолкусунан бери Алекс Перейра кантип өсүп, чемпион болгону таң калтырат. Канча мушкер аракет кылбасын, Перейраны утуш оңой эмес. Анын өмүр баянын жана интервьюларын бул сайттан тап: [url=https://alex-pereira-kg.com/]alex-pereira-kg.com[/url]. Ал уткан сайын Бразилия сыймыктанат.
Ким аны жеңе алат деп ойлойсуңар?
Анын карьерасы али бүтө элек. Анын Adesanya менен болгон салгылашы классика. Анын соккусунан кийин көптөр оңоло албай калган.
Анын жигиттик сапаты өзүнчө сөз. Анын ар бир беттеши көрүүчүлөр үчүн шоу.
Алекс Перейра чындап эле күчтүү мушкер экен, UFCдеги акыркы беттеши укмуш болду. Алекс Перейра UFCде эки салмак категориясында чемпион болду — сыймык! Анын өмүр баянын жана интервьюларын бул сайттан тап: [url=https://alex-pereira-kg.com/]alex-pereira-kg.com[/url]. Ал уткан сайын Бразилия сыймыктанат.
Анын урганынын күчү килограмм менен эсептелет.
Анын тарбиясы жөнөкөй, бирок максаты чоң. Бул жигиттин мүнөзү – темирдей бекем. Анын соккусунан кийин көптөр оңоло албай калган.
Алекс Перейра UFCни өзгөрттү. Перейра дайыма өзүнүн жолун улантат.
Ich kann nicht genug bekommen von NV Casino, es erzeugt eine Energie, die suchtig macht. Es wartet eine Fulle an spannenden Spielen, mit Slots im innovativen Design. Der Kundensupport ist hervorragend, garantiert hochwertige Hilfe. Die Gewinne kommen ohne Verzogerung, manchmal mehr abwechslungsreiche Boni waren willkommen. Insgesamt, NV Casino bietet unvergesslichen Spa? fur Spieler auf der Suche nach Action ! Zusatzlich die Plattform ist optisch ein Highlight, verstarkt die Immersion.
https://playnvcasino.de/|
J’aime l’atmosphere unique de Locowin Casino, il procure une odyssee unique. Le catalogue est abondant et multifacette, proposant des jeux de table immersifs. Accompagne de paris gratuits. L’assistance est efficace et professionnelle, avec une aide precise et rapide. Les retraits sont effectues rapidement, cependant des recompenses additionnelles seraient un avantage. En bref, Locowin Casino est indispensable pour les joueurs pour les fans de casino en ligne ! En bonus l’interface est intuitive et elegante, amplifie le plaisir de jouer. Un avantage supplementaire le programme de fidelite avec des niveaux VIP, assure des transactions fiables.
Aller plus loin|
Je suis stupefait par Locowin Casino, ca transporte dans un univers envoutant. La diversite des titres est epoustouflante, incluant des paris sportifs electrisants. Renforcant l’experience de depart. Le service est operationnel 24/7, avec une assistance exacte et veloce. La procedure est aisee et efficace, par moments des offres plus liberales ajouteraient de la valeur. En synthese, Locowin Casino est une plateforme qui excelle pour les adeptes de sensations intenses ! De surcroit la plateforme est esthetiquement remarquable, ajoute un confort notable. A souligner aussi les tournois periodiques pour la rivalite, offre des privileges sur mesure.
DГ©couvrir toutes les infos|
Je suis completement captive par Casinia Casino, c’est une plateforme qui deborde d’elegance. La selection de jeux est royale, incluant des paris sportifs dynamiques. 100% jusqu’a 500 € + 200 tours gratuits. Les agents repondent avec celerite, offrant des reponses claires. Le processus est simple et elegant, cependant des offres plus genereuses ajouteraient du prestige. En bref, Casinia Casino offre une experience memorable pour les passionnes de jeux modernes ! De plus la plateforme est visuellement somptueuse, donne envie de prolonger l’experience. Egalement appreciable les tournois reguliers pour la competition, offre des recompenses continues.
Approfondir|
Je suis entierement obsede par Locowin Casino, on detecte une vibe folle. Les alternatives sont incroyablement etendues, incluant des paris sportifs electrisants. Associe a des tours gratuits sans wager. L’equipe d’assistance est remarquable, toujours pret a intervenir. Les operations sont solides et veloces, cependant des bonus plus diversifies seraient souhaitables. Tout compte fait, Locowin Casino est essentiel pour les amateurs pour ceux qui parient en crypto ! De surcroit la plateforme est esthetiquement remarquable, ce qui eleve chaque session a un niveau superieur. Egalement notable les paiements securises en crypto, renforce le sens de communaute.
Commencer Г naviguer|
Алекс Перейра UFCде тарых жаратып жатат, бул сөзсүз. Алекс Перейра — чыныгы мисал чыдамкайлык жана эрктүүлүктүн. Эгер Перейранын акыркы беттешин көргүң келсе, кирип чык [url=https://alex-pereira-kg.com/]https://alex-pereira-kg.com[/url]. Алекс Перейра жашоодо көптү башынан өткөргөн.
Анын урганынын күчү килограмм менен эсептелет.
Ал үй-бүлөсүн, балдарын, өлкөсүн сыйлайт. Перейра кикбоксингден баштап, дүйнөлүк октагонго чыкты. Алекс Перейра менен Халил Раунтринин беттеши чынында эле жарылуу болот.
Ал каалаган максатына жеткен. Эгер спортту сүйсөң, Перейранын окуясын оку.
Путешествуйте по Крыму https://м-драйв.рф на джипах! Ай-Петри, Ялта и другие живописные маршруты. Безопасно, интересно и с профессиональными водителями. Настоящий отдых с приключением!
Hi there, You’ve done an excellent job. I will certainly digg it and personally suggest to my friends. I’m confident they’ll be benefited from this site.
казино либет
Бул жигит жөн эле мушкер эмес, чыныгы согуш өнөрүнүн устаты. Канча мушкер аракет кылбасын, Перейраны утуш оңой эмес. Эгер Перейранын акыркы беттешин көргүң келсе, кирип чык [url=https://alex-pereira-kg.com/]https://alex-pereira-kg.com[/url]. Анын Адесанья менен болгон беттеши легендарный болду.
Ал кээде сөз сүйлөбөйт, бирок иш менен далилдейт.
Анын аялын да колдоосу таң калтырат. UFC тарыхында мындай энергия сейрек болот. Анын мурунку беттешин көргөндө чыныгы күчтү сезесиң.
Бүгүн ал дүйнөлүк аренада, бирок жөнөкөй бойдон. Алекс Перейра — күч, эрк жана ишенимдин символу.
Чыныгы мотивация издеген адам Алекс Перейранын жолун көрсүн. Анын тарыхы көп жаштарга мотивация берет. UFC тарыхындагы эң күчтүү мушкерлердин бири тууралуу оку — [url=https://alex-pereira-kg.com/]alex-pereira-kg.com[/url]. Ар бир муштумунда тарых жатат.
Ал кээде сөз сүйлөбөйт, бирок иш менен далилдейт.
Ал үй-бүлөсүн, балдарын, өлкөсүн сыйлайт. Анын жашоосунда дисциплина эң башкысы. Анын мурунку беттешин көргөндө чыныгы күчтү сезесиң.
Бул жигит ар дайым алдыга умтулат. Бул жөн эле мушкер эмес, бул философ.
Нужна карта? иностранные банковские карты MasterCard как оформить зарубежную банковскую карту Visa или MasterCard для россиян в 2025 году. Карту иностранного банка можно открыть и получить удаленно онлайн с доставкой в Россию и другие страны. Зарубежные карты Visa и MasterCard подходят для оплаты за границей. Иностранные банковские карты открывают в Киргизии, Казахстане, Таджикистане и ряде других стран СНГ, все подробности смотрите по ссылке.
Алекс Перейра UFCде тарых жаратып жатат, бул сөзсүз. Мен Перейранын ар бир беттешин күтүп көрөм. Перейра тууралуу баарын бир жерден оку: [url=https://alex-pereira-kg.com/]Перейра Алекс UFC жаңылыктар[/url]. Перейра менен Джон Джонстун беттеши көргүм келет.
Көптөр Перейраны жеңет дешет, бирок ал дайыма жооп берет.
Анын тарбиясы жөнөкөй, бирок максаты чоң. Көрсө, чыныгы чемпиондор көчөдөн да чыгат экен. Менин оюмча, Перейра кайра жеңишке жетет.
Перейра — элдин арасынан чыккан легенда. Перейра дайыма өзүнүн жолун улантат.
Бул жигит жөн эле мушкер эмес, чыныгы согуш өнөрүнүн устаты. Алекс Перейра UFCде эки салмак категориясында чемпион болду — сыймык! Перейра тууралуу баарын бир жерден оку: [url=https://alex-pereira-kg.com/]Перейра Алекс UFC жаңылыктар[/url]. Анын тарыхы көчөдөн башталып, UFC чемпиондугу менен бүттү.
Анын урганынын күчү килограмм менен эсептелет.
Кызыгы, Алекс Перейра Исламды кабыл алган экен. Бул адамдын соккусу таштай катуу. Перейра өзүнүн дараметин кайра-кайра далилдеп келет.
Перейранын жашоосун көрүп, каармандыкты түшүнсө болот. Анын энергиясы баарына жугат.
tripscan трипскан Tripscan трипскан – это повторение названия сервиса на английском и русском языках, что подчеркивает его потенциальную мультиязычность или ориентацию на аудиторию, говорящую как на английском, так и на русском языках. Использование обоих вариантов написания может указывать на необходимость учитывать оба варианта при поиске информации о сервисе в интернете. Описание сервиса остается прежним: планирование путешествий, поиск достопримечательностей, бронирование отелей и обмен опытом между путешественниками.
Металлообработка и металлы https://j-metall.ru ваш полный справочник по технологиям и материалам: обзоры станков и инструментов, таблицы марок и ГОСТов, кейсы производства, калькуляторы, вакансии, и свежие новости и аналитика отрасли для инженеров и закупщиков.
Строительный портал https://repair-house.kiev.ua всё о строительстве, ремонте и архитектуре. Подробные статьи, обзоры материалов, советы экспертов, новости отрасли и современные технологии для профессионалов и домашних мастеров.
Строительный портал https://intellectronics.com.ua источник актуальной информации о строительстве, ремонте и архитектуре. Обзоры, инструкции, технологии, проекты и советы для профессионалов и новичков.
Портал о стройке https://mr.org.ua всё о строительстве, ремонте и дизайне. Статьи, советы экспертов, современные технологии и обзоры материалов. Полезная информация для мастеров, инженеров и владельцев домов.
Актуальный портал https://sinergibumn.com о стройке и ремонте. Современные технологии, материалы, решения для дома и бизнеса. Полезные статьи, инструкции и рекомендации экспертов.
Contemporary digital spaces for grown users offer a selection of interesting opportunities.
These sites are designed for meeting new people and sharing lifestyles.
Users can connect with others who share goals.
Many of these resources encourage safe interaction and welcoming communication.
https://otchizna.info/uncategorized/futanari-porn-exploring-a-popular-niche-in-adult-entertainment/
The interface is usually user-friendly, making it easy to navigate.
Such platforms enable people to express themselves in a free online environment.
Privacy remains an important part of the user experience, with many sites implementing moderation.
Overall, these platforms are created to support adult communication in a safe digital space.
kmspico
Женский портал https://prins.kiev.ua всё о красоте, моде, отношениях, здоровье и саморазвитии. Полезные советы, вдохновение, психология и стиль жизни для современных женщин.
Онлайн женский портал https://replyua.net.ua секреты красоты, стиль, любовь, карьера и семья. Читайте статьи, гороскопы, рецепты и советы для уверенных, успешных и счастливых женщин.
Современный женский https://novaya.com.ua портал о жизни, моде и гармонии. Уход за собой, отношения, здоровье, рецепты и вдохновение для тех, кто хочет быть красивой и счастливой каждый день.
Интересный женский https://muz-hoz.com.ua портал о моде, психологии, любви и красоте. Полезные статьи, тренды, рецепты и лайфхаки. Живи ярко, будь собой и вдохновляйся каждый день!
Женский портал https://z-b-r.org ваш источник идей и вдохновения. Советы по красоте, стилю, отношениям, карьере и дому. Всё, что важно знать современной женщине.
игры [url=https://mailsco.online/]https://mailsco.online/[/url] развлечение способствуют развитию рационального понимания, оптимизируют способности общения.
Онлайн авто портал https://retell.info всё для автолюбителей! Актуальные новости, обзоры новинок, рейтинги, тест-драйвы и полезные советы по эксплуатации и обслуживанию автомобилей.
Автомобильный портал https://autoguide.kyiv.ua для водителей и поклонников авто. Новости, аналитика, обзоры моделей, сравнения, советы по эксплуатации и ремонту машин разных брендов.
Авто портал https://psncodegeneratormiu.org мир машин в одном месте. Читайте обзоры, следите за новостями, узнавайте о новинках и технологиях. Полезный ресурс для автолюбителей и экспертов.
Авто портал https://bestsport.com.ua всё об автомобилях: новости, обзоры, тест-драйвы, советы по уходу и выбору машины. Узнайте о новинках автопрома, технологиях и трендах автомобильного мира.
Современный авто портал https://necin.com.ua мир автомобилей в одном месте. Тест-драйвы, сравнения, новости автопрома и советы экспертов. Будь в курсе последних тенденций автоиндустрии
J’adore l’atmosphere de Bingoal Casino, ca procure un thrill incomparable. La diversite des titres est stupefiante, incluant des paris sportifs palpitants. Accompagne de paris gratuits. Le service est disponible 24/7, toujours disponible pour assister. Les paiements sont securises et fluides, de temps a autre quelques tours gratuits supplementaires seraient bienvenus. Pour conclure, Bingoal Casino est une plateforme qui impressionne pour ceux qui aiment parier en crypto ! Ajoutons que le design est moderne et fluide, facilite une immersion totale. A souligner les paiements securises en crypto, qui booste l’engagement.
Voir les dГ©tails|
Ich bin beeindruckt von SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Es wartet eine Fulle spannender Optionen, mit innovativen Slots und fesselnden Designs. Der Service ist von hoher Qualitat, verfugbar rund um die Uhr. Die Auszahlungen sind ultraschnell, dennoch mehr abwechslungsreiche Boni waren super. Global gesehen, SpinBetter Casino garantiert hochsten Spa? fur Spieler auf der Suche nach Action ! Zusatzlich die Navigation ist kinderleicht, gibt den Anreiz, langer zu bleiben. Zusatzlich zu beachten die Vielfalt an Zahlungsmethoden, die Flexibilitat bieten.
spinbettercasino.de|
J’aime l’environnement distinct de Bingoal Casino, on percoit une vitalite dechainee. Les alternatives sont etonnamment etendues, avec des slots innovants et thematises. Le bonus d’inscription est seduisant. L’aide est performante et experte, proposant des reponses limpides. Les paiements sont proteges et lisses, bien que plus de promotions frequentes seraient un atout. En synthese, Bingoal Casino merite une exploration approfondie pour les aficionados de jeux contemporains ! Ajoutons que l’interface est intuitive et raffinee, ajoute un confort notable. Un plus significatif les evenements communautaires captivants, offre des privileges sur mesure.
AccГ©der maintenant|
Je suis epate par Locowin Casino, ca transporte dans un monde captivant. La selection de jeux est phenomenale, proposant des jeux de table immersifs. Amplifiant l’experience initiale. Le service est disponible 24/7, garantissant un support de qualite. Le processus est simple et efficace, neanmoins plus de promos regulieres seraient top. Au final, Locowin Casino vaut largement le detour pour les amateurs de sensations fortes ! Notons aussi la plateforme est visuellement top, ce qui rend chaque session encore plus fun. Un plus non negligeable les tournois reguliers pour la competition, qui booste l’engagement.
DГ©couvrir dГЁs maintenant|
J’adore l’aura princiere de Casinia Casino, il offre une aventure imperiale. Il y a une profusion de jeux captivants, comprenant des jeux compatibles avec les cryptos. Avec un Bonus Crab exclusif. Les agents repondent avec une rapidite fulgurante, joignable a toute heure. Les paiements sont securises et fluides, de temps a autre plus de promos frequentes brilleraient davantage. Dans l’ensemble, Casinia Casino fournit une experience inoubliable pour les amateurs de casino en ligne ! A noter le design est opulent et envoutant, incite a prolonger l’experience. Un avantage notable les evenements communautaires engageants, cree une communaute vibrante.
Voir les mises Г jour|
Je suis totalement seduit par Casinia Casino, on ressent une energie noble. Les options sont vastes comme un royaume, avec des slots thematiques et innovants. Amplifiant le plaisir de jeu. Le suivi est impeccable, toujours pret a servir. Le processus est simple et elegant, bien que des recompenses additionnelles seraient royales. En bref, Casinia Casino offre une experience memorable pour les amateurs de sensations fortes ! Ajoutons que la navigation est simple et agreable, amplifie le plaisir de jouer. Particulierement interessant les options de paris sportifs variees, offre des recompenses continues.
Explorer le guide|
Je suis accro a Locowin Casino, c’est une plateforme qui explose d’energie. La diversite des titres est epoustouflante, incluant des paris sportifs electrisants. Renforcant l’experience de depart. Le service est operationnel 24/7, proposant des reponses limpides. La procedure est aisee et efficace, de temps a autre des bonus plus diversifies seraient souhaitables. En synthese, Locowin Casino garantit du divertissement constant pour ceux qui parient en crypto ! A mentionner le design est contemporain et lisse, facilite une immersion complete. Un plus significatif le programme VIP avec des niveaux exclusifs, propose des recompenses permanentes.
Lire la suite|
Портал про стройку https://dcsms.uzhgorod.ua всё о строительстве, ремонте и дизайне. Полезные советы, статьи, технологии, материалы и оборудование. Узнайте о современных решениях для дома и бизнеса.
Строительный портал https://msc.com.ua о ремонте, дизайне и технологиях. Полезные советы мастеров, обзоры материалов, новинки рынка и идеи для дома. Всё о стройке — от фундамента до отделки. Учись, строй и вдохновляйся вместе с нами!
Портал про стройку https://keravin.com.ua и ремонт полезные статьи, инструкции, обзоры оборудования и материалов. Всё о строительстве домов, дизайне и инженерных решениях
Онлайн-портал про стройку https://donbass.org.ua и ремонт. Новости, проекты, инструкции, обзоры материалов и технологий. Всё, что нужно знать о современном строительстве и архитектуре.
Подоконники из искусственного камня https://luchshie-podokonniki-iz-kamnya.ru в Москве. Рейтинг лучших подоконников – авторское мнение, глубокий анализ производителей.
I’m stunned by Wazamba Casino, it’s a saga that whispers with secrets. The varieties are comprehensive and layered, including over 5,000 offerings from leading creators. Paired with 200 gratis rotations. Promoting continuous enjoyment. Disbursements are managed efficiently, although supplemental rotations could advance it. Collectively , Wazamba Casino furnishes superior recreation for wagering lovers ! Moreover the architecture is creatively striking, fortifying absorption in play. A gain is nurturing collective spirit, escalating participation.
wazambagr.com|
connectwiththeworldnow – Their mission seems bold, site feels ambitious yet hopeful.
I’m blown away by Astronaut Crash by 100HP Gaming, it offers a stellar strategic challenge. The provably fair system builds trust, featuring a rocket that soars with rising multipliers. Volatility keeps things exciting and fair. Support is lightning-fast and helpful, providing clear multiplier histories. The cash-out feature saves fortunes, occasionally additional themes beyond space. Wrapping it up, Astronaut Crash delivers proven fair thrills for crash game fans ! Worth mentioning the sound design amps the tension, heightening the launch anticipation. Notably tournaments for competitive crashes, ensures fast and fee-free payouts.
Astronaut Crash Game|
Советы по строительству https://vodocar.com.ua и ремонту своими руками. Пошаговые инструкции, современные технологии, идеи для дома и участка. Мы поможем сделать ремонт проще, а строительство — надёжнее!
Сайт о строительстве https://valkbolos.com и ремонте домов, квартир и дач. Полезные советы мастеров, подбор материалов, дизайн-идеи, инструкции и обзоры инструментов. Всё, что нужно для качественного ремонта и современного строительства!
Полезный сайт https://stroy-portal.kyiv.ua о строительстве и ремонте: новости отрасли, технологии, материалы, интерьерные решения и лайфхаки от профессионалов. Всё для тех, кто строит, ремонтирует и создаёт уют.
Строительный сайт https://teplo.zt.ua для тех, кто создаёт дом своей мечты. Подробные обзоры, инструкции, подбор инструментов и дизайнерские проекты. Всё о ремонте и строительстве в одном месте.
Информационный портал https://smallbusiness.dp.ua про строительство, ремонт и интерьер. Свежие новости отрасли, обзоры технологий и полезные лайфхаки. Всё, что нужно знать о стройке и благоустройстве жилья в одном месте!
connectwiththeworldnow – The brand feels urgent, visuals add strength to the narrative.
сколько стоит машина maserati купить volvo – это приобрести автомобиль с высоким уровнем безопасности, комфорта и инновационных технологий, сочетающий в себе скандинавский дизайн и надежность.
Строим и ремонтируем https://buildingtips.kyiv.ua своими руками! Инструкции, советы, видеоуроки и лайфхаки для дома и дачи. Узнай, как сделать ремонт качественно и сэкономить бюджет.
Энциклопедия строительства https://kero.com.ua и ремонта: материалы, технологии, интерьерные решения и практические рекомендации. От фундамента до декора — всё, что нужно знать домовладельцу.
Пошаговые советы https://tsentralnyi.volyn.ua по строительству и ремонту. Узнай, как выбрать материалы, рассчитать бюджет и избежать ошибок. Простые решения для сложных задач — строим и ремонтируем с уверенностью!
Новостной портал https://kiev-online.com.ua с проверенной информацией. Свежие события, аналитика, репортажи и интервью. Узнавайте новости первыми — достоверно, быстро и без лишнего шума.
Главные новости дня https://sevsovet.com.ua эксклюзивные материалы, горячие темы и аналитика. Мы рассказываем то, что действительно важно. Будь в курсе вместе с нашим новостным порталом!
Флойд Мейвезер ар дайым мыкты формада болот. Флойд Мейвезер жеңиштеринин саны боюнча рекорд койгон. Кызыктуу статистика жана жаңылыктар [url=https://floyd-mayweather-kg.com/]Флойд Мейвезер статистика[/url] бөлүмүндө топтолгон.
Флойд Мейвезерди көрүү ар бир спорт күйөрманы үчүн майрам. Анын рекорддору бүгүнкү күнгө чейин сакталган. Флойд Мейвезердин карьерасы көп жылдык эмгектин натыйжасы.
Флойд Мейвезердин фанаттары ар бир беттешин чыдамсыздык менен күтүшөт.
Анын жашоосу мотивациянын булагы. Флойд Мейвезер ар дайым өзүнө ишенген. Анын ар бир интервьюсу чоң кызыгуу жаратат.
Строим и ремонтируем https://srk.kiev.ua грамотно! Инструкции, пошаговые советы, видеоуроки и экспертные рекомендации. Узнай, как сделать ремонт качественно и сэкономить без потери результата.
Строительный портал https://sitetime.kiev.ua для мастеров и подрядчиков. Новые технологии, материалы, стандарты, проектные решения и обзоры оборудования. Всё, что нужно специалистам стройиндустрии.
Обустраивайте дом https://stroysam.kyiv.ua со вкусом! Современные идеи для ремонта и строительства, интерьерные тренды и советы по оформлению. Создайте стильное и уютное пространство своими руками.
Сайт о стройке https://samozahist.org.ua и ремонте для всех, кто любит уют и порядок. Расскажем, как выбрать материалы, обновить интерьер и избежать ошибок при ремонте. Всё просто, полезно и по делу.
Как построить https://rus3edin.org.ua и отремонтировать своими руками? Пошаговые инструкции, простые советы и подбор инструментов. Делаем ремонт доступным и понятным для каждого!
Флойд Мейвезердин карьерасы тарыхта өзгөчө орунда. Флойд Мейвезердин акыркы жаңылыктары ар бир күйөрманды кызыктырат. Жаңы жаңылыктарды окуу үчүн [url=https://floyd-mayweather-kg.com/]floyd-mayweather-kg.com[/url] барагына өтүңүз.
Флойд Мейвезердин карьерасы көп жылдарга созулган. Анын рекорддору бүгүнкү күнгө чейин сакталган. Анын акыркы жаңылыктарын укпай калган адам жок.
Анын ар бир интервьюсу көңүл борборунда.
Анын карьерасы ар бир жаш спортчу үчүн үлгү. Флойд Мейвезер ар бир жаш спортчуга үлгү. Анын жашоосу жана ийгилиги мотивация берет.
https://wordpress.org/support/topic/can-i-make-ai-chatbot-automatically-open/
Мужской сайт https://rkas.org.ua о жизни без компромиссов: спорт, путешествия, техника, карьера и отношения. Для тех, кто ценит свободу, силу и уверенность в себе.
Портал для автомобилистов https://translit.com.ua от выбора машины до профессионального ремонта. Читайте обзоры авто, новости автоспорта, сравнивайте цены и характеристики. Форум автолюбителей, советы экспертов и свежие предложения автосалонов.
Сайт для женщин https://oun-upa.org.ua которые ценят себя и жизнь. Мода, советы по уходу, любовь, семья, вдохновение и развитие. Найди идеи для новых свершений и будь самой собой в мире, где важно быть уникальной!
Мужской онлайн-журнал https://cruiser.com.ua о современных трендах, технологиях и саморазвитии. Мы пишем о том, что важно мужчине — от мотивации и здоровья до отдыха и финансов.
Ваш гид в мире https://nerjalivingspace.com автомобилей! Ежедневные авто новости, рейтинги, тест-драйвы и советы по эксплуатации. Найдите идеальный автомобиль, узнайте о страховании, кредитах и тюнинге.
Флойд Мейвезер дүйнөдөгү эң белгилүү мушкерлердин бири. Флойд Мейвезер эч качан жеңилбеген мушкер катары белгилүү. Бокс күйөрмандары үчүн кызыктуу фактылар [url=https://floyd-mayweather-kg.com/]https://floyd-mayweather-kg.com/[/url] порталында жайгашкан.
Анын фамилиясы дүйнөлүк брендге айланган. Анын ийгилиги ар бир спортчуга мотивация. Флойд Мейвезер ар дайым өзүн мыкты формада кармаган.
Флойд Мейвезер ар бир кадамын ойлонуп жасайт.
Флойд Мейвезер дүйнөдөгү эң жогорку кирешелүү спортчу. Флойд Мейвезер ар дайым өзүнө ишенген. Флойд Мейвезер ар дайым жаңы рекорддорду жарата берет.
J’aime l’atmosphere unique de Bingoal Casino, ca immerse dans un monde fascinant. L’eventail de jeux est spectaculaire, comprenant des jeux optimises pour les cryptos. Doublement des depots jusqu’a 200 €. Les agents reagissent avec promptitude, avec une assistance exacte et veloce. Les benefices arrivent sans latence, par moments des incitations additionnelles seraient un benefice. En synthese, Bingoal Casino merite une exploration approfondie pour les adeptes de sensations intenses ! Ajoutons que la navigation est simple et engageante, ce qui eleve chaque session a un niveau superieur. Un plus significatif les paiements securises en crypto, offre des privileges sur mesure.
Explorer le contenu|
Ich bin komplett hin und weg von SpinBetter Casino, es erzeugt eine Spielenergie, die fesselt. Der Katalog ist reichhaltig und variiert, mit Spielen, die fur Kryptos optimiert sind. Der Support ist 24/7 erreichbar, garantiert top Hilfe. Die Zahlungen sind sicher und smooth, gelegentlich mehr abwechslungsreiche Boni waren super. Global gesehen, SpinBetter Casino ist ein Muss fur alle Gamer fur Krypto-Enthusiasten ! Zusatzlich die Interface ist intuitiv und modern, fugt Magie hinzu. Zusatzlich zu beachten die Vielfalt an Zahlungsmethoden, die Vertrauen schaffen.
spinbettercasino.de|
J’aime l’environnement distinct de Bingoal Casino, il offre une odyssee incomparable. L’eventail de jeux est extraordinaire, proposant des jeux de table immersifs. Associe a des paris gratuits. Le suivi est exemplaire, accessible a tout instant. Les operations sont solides et veloces, cependant des bonus plus diversifies seraient souhaitables. En synthese, Bingoal Casino merite une exploration approfondie pour les adeptes de sensations intenses ! Par ailleurs le design est contemporain et lisse, ajoute un confort notable. Un autre avantage cle les paiements securises en crypto, garantit des transactions securisees.
Voir la page|
J’adore l’ambiance de Locowin Casino, c’est une plateforme qui bouillonne d’energie. Il y a une multitude de jeux excitants, incluant des paris sportifs palpitants. Pour un demarrage en force. L’assistance est efficace et pro, garantissant un support de qualite. Les retraits sont ultra-rapides, mais plus de promos regulieres seraient top. En resume, Locowin Casino est un incontournable pour les joueurs pour les passionnes de jeux modernes ! En prime la plateforme est visuellement top, ce qui rend chaque session encore plus fun. Particulierement interessant les evenements communautaires engageants, offre des recompenses continues.
AccГ©der au site|
J’ai une passion debordante pour Casinia Casino, ca transporte dans un palais scintillant. Le repertoire est riche et multifacette, avec des slots aux designs audacieux et thematiques. Avec un Bonus Crab unique pour debuter. Le service est disponible 24/7, joignable a toute heure. Les transactions sont fiables et rapides, parfois quelques tours gratuits supplementaires seraient lumineux. En bref, Casinia Casino est une plateforme qui illumine le jeu pour les joueurs en quete d’eclat ! Par ailleurs la navigation est intuitive comme une lueur, ajoute une touche de splendeur. Un avantage notable les paiements securises en crypto, offre des privileges continus.
Commencer Г lire|
Je suis captive par Casinia Casino, c’est une plateforme qui rayonne de luxe. Il y a une abondance de jeux captivants, offrant des sessions live immersives. Renforcant votre capital initial. Le support client est imperial, offrant des reponses claires. Les retraits sont rapides comme l’eclair, cependant des offres plus genereuses ajouteraient du prestige. Pour conclure, Casinia Casino garantit du fun a chaque instant pour les passionnes de jeux modernes ! De plus le design est moderne et fluide, amplifie le plaisir de jouer. Particulierement interessant les tournois reguliers pour la competition, offre des recompenses continues.
Commencer Г explorer|
Je suis accro a Locowin Casino, on ressent une vibe delirante. La variete des titres est impressionnante, avec des slots au design innovant. Pour un demarrage en force. Le support client est exceptionnel, offrant des reponses claires. Les retraits sont ultra-rapides, neanmoins des bonus plus varies seraient bienvenus. Pour conclure, Locowin Casino offre une experience memorable pour ceux qui aiment parier en crypto ! Cerise sur le gateau le design est moderne et fluide, ce qui rend chaque session encore plus fun. Particulierement interessant les evenements communautaires engageants, qui booste l’engagement.
Trouver les infos|
Флойд Мейвезер дүйнөдөгү эң белгилүү мушкерлердин бири. Флойд Мейвезердин акыркы беттеши көп талкуу жараткан. Бокс күйөрмандары үчүн кызыктуу фактылар [url=https://floyd-mayweather-kg.com/]https://floyd-mayweather-kg.com/[/url] порталында жайгашкан.
Анын фамилиясы дүйнөлүк брендге айланган. Флойд Мейвезер – мыкты стратегиянын ээси. Флойд Мейвезердин жашоосу – мотивация.
Флойд Мейвезер ар бир кадамын ойлонуп жасайт.
Анын акыркы беттеши чоң окуя болгон. Флойд Мейвезердин жашоосу ар дайым элдин көңүлүндө. Флойд Мейвезердин ар бир жаңылыгы спорт дүйнөсүндө жаңылык.
I’m astonished by Wazamba Casino, it crafts a captivating saga. The repository is immensely comprehensive, incorporating live sessions for authentic interaction. Matching initial funds up to €500. Assistance team is superb. Profits are delivered swiftly, from time to time supplementary perks would excel. Ending with, Wazamba Casino merits investigation for digital currency admirers ! Also the framework is artistically impressive, magnifying each episode’s appeal. Additional benefit reliable crypto handling methods, guaranteeing safe exchanges.
wazambagr.com|
Флойд Мейвезер спорт дүйнөсүндөгү эң бай спортчу. Флойд Мейвезердин акыркы жаңылыктары ар бир күйөрманды кызыктырат. Флойд Мейвезердин карьерасы жана акыркы жаңылыктары тууралуу көбүрөөк маалыматты [url=https://floyd-mayweather-kg.com/]Флойд Мейвезер[/url] сайтынан таба аласыз.
Флойд Мейвезердин ар бир беттеши чыныгы спектакль. Флойд Мейвезер ар бир беттешинде тактиканы мыкты колдонот. Флойд Мейвезердин карьерасы көп жылдык эмгектин натыйжасы.
Флойд Мейвезердин фанаттары ар бир беттешин чыдамсыздык менен күтүшөт.
Флойд Мейвезердин тарыхтагы орду чоң. Флойд Мейвезердин жашоосу ар дайым элдин көңүлүндө. Анын жашоосу жана ийгилиги мотивация берет.
Портал о дизайне https://sculptureproject.org.ua интерьеров и пространства. Идеи, тренды, проекты и вдохновение для дома, офиса и общественных мест. Советы дизайнеров и примеры стильных решений каждый день.
Строительный сайт https://okna-k.com.ua для профессионалов и новичков. Новости отрасли, обзоры материалов, технологии строительства и ремонта, советы мастеров и пошаговые инструкции для качественного результата.
Сайт о металлах https://metalprotection.com.ua и металлообработке: виды металлов, сплавы, технологии обработки, оборудование и новости отрасли. Всё для специалистов и профессионалов металлургии.
Главный автопортал страны https://nmiu.org.ua всё об автомобилях в одном месте! Новости, обзоры, советы, автообъявления, страхование, ТО и сервис. Для водителей, механиков и просто любителей машин.
Женский онлайн-журнал https://rosetti.com.ua о стиле, здоровье и семье. Новости моды, советы экспертов, тренды красоты и секреты счастья. Всё, что важно и интересно женщинам любого возраста.
social stream
I can’t stop raving about Astronaut Crash by 100HP Gaming, it presents a masterful mix of luck and timing. Bet ranges cater to every wallet size, with auto-bet for hands-free excitement. Balanced volatility for steady thrills. Forums buzzing with player tactics, eager to troubleshoot mid-flight. Flows are dependable and speedy, still weekly challenges could ramp up fun. All told, Astronaut Crash sparks endless stellar sessions for multiplier maniacs ! On that note controls are beginner-proof, streamlining stake tweaks. Highlight leaderboards for rivalry, facilitates low-cost transfers.
Astronaut Crash Game|
Студия ремонта https://anti-orange.com.ua квартир и домов. Выполняем ремонт под ключ, дизайн-проекты, отделочные и инженерные работы. Качество, сроки и индивидуальный подход к каждому клиенту.
Туристический портал https://feokurort.com.ua для любителей путешествий! Страны, маршруты, достопримечательности, советы и лайфхаки. Планируйте отдых, находите вдохновение и открывайте мир вместе с нами.
Флойд Мейвезер ар дайым мыкты формада болот. Флойд Мейвезер жеңиштеринин саны боюнча рекорд койгон. Бардык жаңылыктарды жана видео материалдарды [url=https://floyd-mayweather-kg.com/]www.floyd-mayweather-kg.com[/url] сайтынан көрүңүз.
Флойд Мейвезердин жашоосу мотивация берет. Анын ийгилиги ар бир спортчуга мотивация. Флойд Мейвезердин жашоосу – мотивация.
Флойд Мейвезердин акыркы жаңылыктары спорт дүйнөсүн дүрбөлөңгө салат.
Флойд Мейвезердин рекорддору таң калтырат. Флойд Мейвезердин атын угуу эле сыймык. Анын ар бир интервьюсу чоң кызыгуу жаратат.
Флойд Мейвезер ар бир беттешинде жогорку деңгээлди көрсөтөт. Флойд Мейвезердин акыркы жаңылыктары ар бир күйөрманды кызыктырат. Кызыктуу статистика жана жаңылыктар [url=https://floyd-mayweather-kg.com/]Флойд Мейвезер статистика[/url] бөлүмүндө топтолгон.
Флойд Мейвезер жаш кезинен тарта бокс менен алектенген. Флойд Мейвезер спорт тарыхына кирген аттардын бири. Анын акыркы жаңылыктарын укпай калган адам жок.
Флойд Мейвезер – тартиптин жана күчтүн символу.
Флойд Мейвезер дүйнөдөгү эң жогорку кирешелүү спортчу. Анын карьерасы спорт тарыхында алтын тамга менен жазылган. Флойд Мейвезер – күч, ылдамдык жана акылдын айкалышы.
Ich habe einen totalen Hang zu SpinBetter Casino, es ist eine Erfahrung, die wie ein Wirbelsturm pulsiert. Es wartet eine Fulle spannender Optionen, mit dynamischen Tischspielen. Der Support ist 24/7 erreichbar, garantiert top Hilfe. Die Gewinne kommen prompt, dennoch zusatzliche Freispiele waren ein Highlight. In Kurze, SpinBetter Casino ist eine Plattform, die uberzeugt fur Spieler auf der Suche nach Action ! Au?erdem die Navigation ist kinderleicht, verstarkt die Immersion. Ein weiterer Vorteil die Vielfalt an Zahlungsmethoden, die Flexibilitat bieten.
spinbettercasino.de|
Je suis bluffe par Casinia Casino, ca transporte dans un palais enchanteur. Les options sont vastes comme un royaume, incluant des paris sportifs dynamiques. Plus un Bonus Crab pour demarrer. Le support client est imperial, toujours pret a servir. Le processus est simple et elegant, parfois des bonus plus varies seraient apprecies. Dans l’ensemble, Casinia Casino est un incontournable pour les joueurs pour ceux qui aiment parier en crypto ! En prime la navigation est simple et agreable, facilite une immersion totale. Un plus non negligeable le programme VIP avec 5 niveaux, qui booste l’engagement.
VГ©rifier plus|
melbet – paris sportif foot africain
pronostic foot gratuit 1xbet afrique apk
Студия дизайна https://bathen.rv.ua интерьеров и архитектурных решений. Создаём стильные, функциональные и гармоничные пространства. Индивидуальный подход, авторские проекты и внимание к деталям.
Ремонт и строительство https://fmsu.org.ua без лишних сложностей! Подробные статьи, обзоры инструментов, лайфхаки и практические советы. Мы поможем построить, отремонтировать и обустроить ваш дом.
1xbet cameroun apk 1xbet africain
Je suis hypnotise par Casinia Casino, c’est une plateforme qui scintille de grandeur. Il y a une profusion de jeux captivants, comprenant des jeux compatibles avec les cryptos. Renforcant votre tresor initial. Le support client est royal, garantissant un service princier. Les paiements sont securises et fluides, neanmoins des recompenses additionnelles seraient imperiales. Au final, Casinia Casino offre un divertissement constant pour les passionnes de frissons grandioses ! Ajoutons que la plateforme scintille comme un palais, incite a prolonger l’experience. Egalement remarquable les tournois reguliers pour la rivalite, garantit des transactions fiables.
Ouvrir ici|
J’ai un faible pour Casinia Casino, c’est une plateforme qui rayonne de luxe. Le catalogue est opulent et diversifie, offrant des sessions live immersives. Le bonus de bienvenue est genereux. Le support client est imperial, offrant des reponses claires. Le processus est simple et elegant, parfois des offres plus genereuses ajouteraient du prestige. Pour conclure, Casinia Casino est un incontournable pour les joueurs pour les joueurs en quete d’excitation ! Notons aussi le site est rapide et attrayant, facilite une immersion totale. Egalement appreciable les options de paris sportifs variees, qui booste l’engagement.
DГ©couvrir davantage|
Je suis surpris par Locowin Casino, ca fournit un plaisir intense. Il y a une profusion de jeux captivants, proposant des jeux de table immersifs. Le bonus d’accueil est attractif. L’aide est performante et experte, assurant un support premium. Les benefices arrivent sans latence, mais plus de promotions frequentes seraient un atout. Tout compte fait, Locowin Casino fournit une experience ineffacable pour les enthousiastes de casino en ligne ! En outre le site est veloce et seduisant, facilite une immersion complete. Particulierement attractif les paiements securises en crypto, offre des privileges sur mesure.
Aller pour les dГ©tails|
connectwiththeworldnow – The brand feels urgent, visuals add strength to the narrative.
Wow, superb weblog structure! How lengthy have you ever been blogging for? you make running a blog look easy. The overall look of your site is great, as neatly as the content!
официальный сайт kra41
I can’t get enough of Wazamba Casino, it unleashes a special vibe. The library is truly enormous, with sports betting to diversify. Boosting your playtime. Round-the-clock availability. Transactions are safe and efficient, yet expanded bonus varieties would be great. To wrap up, Wazamba Casino stands out as a superior platform for adventure seekers ! Moreover the visuals are captivating and immersive, streamlining user experience. Particularly impressive regular tournaments for competition, boosting player retention.
https://wazambagr.com/|
Современная студия дизайна https://bconline.com.ua архитектура, интерьер, декор. Мы создаём пространства, где технологии сочетаются с красотой, а стиль — с удобством.
pronostics du foot afrik foot pronostic
pronostic foot gratuit telecharger 1xbet apk
telecharger 1xbet 1xbet afrique apk
Создавайте дом https://it-cifra.com.ua своей мечты! Всё о строительстве, ремонте и дизайне интерьера. Идеи, проекты, фото и инструкции — вдохновляйтесь и воплощайте задуманное легко и с удовольствием.
bs2best зеркало Что, еще не слышал о сенсации, сотрясающей глубины даркнета? Blacksprut – это не просто торговая марка. Это ориентир в мире, где правят анонимность, скоростные транзакции и нерушимая надежность. Спеши на bs2best.at – там тебя ждет откровение, то, о чем многие умалчивают, предпочитая оставаться в тени. Ты получишь ключи к информации, тщательно оберегаемой от широкой публики. Только для тех, кто посвящен в суть вещей. Полное отсутствие следов. Никаких компромиссов. Лишь совершенство, имя которому Blacksprut. Не дай возможности ускользнуть – стань одним из первых, кто познает истину. bs2best.at уже распахнул свои объятия. Готов ли ты к встрече с реальностью, которая шокирует?
ascendgrid – Great name, hope their content or services match.
truelaunch – Clear explanations, made complex stuff easier to get.
brandvision – The visuals and typography are sharp and really appealing.
Мир архитектуры https://vineyardartdecor.com и дизайна в одном месте! Лучшие идеи, проекты и вдохновение для дома, офиса и города. Узнай, как создаются красивые и функциональные пространства.
Ich habe einen totalen Hang zu SpinBetter Casino, es fuhlt sich an wie ein Strudel aus Freude. Das Angebot an Spielen ist phanomenal, mit aufregenden Sportwetten. Der Support ist 24/7 erreichbar, verfugbar rund um die Uhr. Der Ablauf ist unkompliziert, dennoch die Offers konnten gro?zugiger ausfallen. Alles in allem, SpinBetter Casino ist absolut empfehlenswert fur Casino-Liebhaber ! Daruber hinaus die Interface ist intuitiv und modern, gibt den Anreiz, langer zu bleiben. Zusatzlich zu beachten die Sicherheit der Daten, die den Einstieg erleichtern.
spinbettercasino.de|
Je suis emerveille par Locowin Casino, il procure une odyssee unique. Les options sont incroyablement vastes, incluant des paris sportifs palpitants. Accompagne de paris gratuits. L’equipe de support est remarquable, toujours disponible pour assister. Les gains arrivent sans retard, cependant des recompenses additionnelles seraient un avantage. En resume, Locowin Casino merite une visite pour les passionnes de jeux modernes ! Par ailleurs la navigation est simple et plaisante, facilite une immersion totale. A souligner le programme de fidelite avec des niveaux VIP, propose des avantages personnalises.
VГ©rifier la liste complГЁte|
J’apprecie l’environnement de Locowin Casino, il delivre une experience unique. La diversite des titres est epoustouflante, incluant des paris sportifs electrisants. Pour un lancement puissant. Le service est operationnel 24/7, accessible a tout instant. La procedure est aisee et efficace, bien que quelques tours gratuits supplementaires seraient apprecies. Globalement, Locowin Casino garantit du divertissement constant pour les joueurs a la recherche d’aventure ! Par ailleurs la navigation est simple et engageante, intensifie le plaisir du jeu. A souligner aussi le programme VIP avec des niveaux exclusifs, qui stimule l’engagement.
Essayer ceci|
Je suis stupefait par Locowin Casino, ca fournit un plaisir intense. La diversite des titres est epoustouflante, proposant des jeux de table immersifs. Pour un lancement puissant. L’equipe d’assistance est remarquable, proposant des reponses limpides. Les paiements sont proteges et lisses, bien que des bonus plus diversifies seraient souhaitables. Pour synthetiser, Locowin Casino garantit du divertissement constant pour les adeptes de sensations intenses ! Ajoutons que la plateforme est esthetiquement remarquable, stimule le desir de revenir. Egalement notable les tournois periodiques pour la rivalite, propose des recompenses permanentes.
Cliquez ici|
valuevision – Found some good sections, wish there was more detailed content.
growthverse – Navigation is seamless, and the visuals are appealing.
I’m amazed by Wazamba Casino, it’s an adventure that throbs with excitement. The selection of games is phenomenal, including slots with tribal themes. Plus 200 free spins to start strong. Ensuring smooth gameplay. Payments are secure and reliable, though lower wagering requirements would be ideal. In conclusion, Wazamba Casino is worth exploring for those who enjoy crypto ! Additionally navigation is effortless, simplifies the overall experience. Especially great the loyalty program with masks, elevating the engagement.
wazambagr.com|
skyportal – I always find helpful info, navigation is smooth too.
trendforge – The name suggests being ahead of trends; that’s appealing.
smartgrid – Very little visible now; probably under construction.
Estou completamente encantado por PlayPIX Casino, e uma plataforma que pulsa com energia festiva. As opcoes sao vastas como um desfile, com sessoes ao vivo dinamicas. 100% ate €500 + 200 rodadas gratis. Os agentes respondem com rapidez, com suporte preciso e rapido. Os pagamentos sao seguros e fluidos, no entanto mais promocoes regulares seriam otimas. Para concluir, PlayPIX Casino garante diversao a cada momento para fas de cassino online ! Adicionalmente a plataforma e visualmente espetacular, facilita uma imersao total. Um diferencial importante os torneios regulares para competicao, assegura transacoes confiaveis.
Ver os detalhes|
quantumreach – Bookmarking this — looks like a future go-to site.
boldimpact – The domain looks strong, hoping content is up soon.
alphaunity – Loading speed is good, didn’t wait long for pages.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
bluetrail – Found some useful resources here- helpful for my projects.
sharpbridge – The color scheme is nice, very calming on the eyes.
solidvision – Clean branding, simple domain—can work well.
elitegrowth – Offers valuable insights, and the interface is top-notch.
cloudmatrix – Found exactly what I was looking for, very intuitive.
goldbridge – The domain looks solid, hoping the content matches up well.
fastgrowth – The content is engaging, and the site is responsive.
maxvision – The content is engaging, and the site is responsive.
greenmotion – Found exactly what I was looking for, very intuitive.
zenithlabs – Offers valuable insights, and the interface is top-notch.
thinkbeyondtech – Found exactly what I was looking for, very intuitive.
nextlayer – Found exactly what I was looking for, very intuitive.
boldnetwork – Found this by chance, curious what kind of content they’ll share.
https://b2tsite3.cc
alihidaer2313 – I like the clean layout, content seems purposeful and honest.
Je suis completement fou de Locowin Casino, on ressent une vibe delirante. Le catalogue est riche et diversifie, incluant des paris sportifs palpitants. Pour un demarrage en force. Le support client est exceptionnel, toujours pret a aider. Les paiements sont securises et fluides, mais plus de promos regulieres seraient top. Pour conclure, Locowin Casino vaut largement le detour pour les fans de casino en ligne ! Notons aussi l’interface est intuitive et stylee, facilite une immersion totale. Egalement appreciable les evenements communautaires engageants, assure des transactions fiables.
Voir les nouveautГ©s|
Автоматические гаражные ворота давно перестали быть роскошью и стали необходимым элементом комфортной жизни. Наши автоматические ворота сочетают надёжность проверенных европейских механизмов с элегантным дизайном, который гармонично впишется в архитектуру любого здания. Мы предлагаем полный цикл услуг: от профессиональной консультации и точного замера до установки под ключ и гарантийного обслуживания. Доверьте безопасность своего дома профессионалам — получите бесплатный расчёт стоимости уже сегодня: Гаражные ворота
J’ai un engouement sincere pour Locowin Casino, ca fournit un plaisir intense. Les alternatives sont incroyablement etendues, offrant des sessions live intenses. Renforcant l’experience de depart. L’equipe d’assistance est remarquable, accessible a tout instant. Les benefices arrivent sans latence, de temps a autre des offres plus liberales ajouteraient de la valeur. Globalement, Locowin Casino fournit une experience ineffacable pour les adeptes de sensations intenses ! Par ailleurs la plateforme est esthetiquement remarquable, ajoute un confort notable. Egalement notable les evenements communautaires captivants, qui stimule l’engagement.
DГ©couvrir le site complet|
Заботьтесь о здоровье сосудов ног с профессионалом! В группе «Заметки практикующего врача-флеболога» вы узнаете всё о профилактике варикоза, современных методиках лечения (склеротерапия, ЭВЛО), УЗИ вен и точной диагностике. Доверяйте опытному врачу — ваши ноги заслуживают лучшего: https://phlebology-blog.ru/
J’aime l’atmosphere de Locowin Casino, c’est une plateforme qui explose d’energie. La gamme de jeux est spectaculaire, offrant des sessions live intenses. Renforcant l’experience de depart. Le service est operationnel 24/7, assurant un support premium. Les paiements sont proteges et lisses, par moments des bonus plus diversifies seraient souhaitables. En fin de compte, Locowin Casino fournit une experience ineffacable pour les joueurs a la recherche d’aventure ! En outre le site est veloce et seduisant, stimule le desir de revenir. Particulierement attractif les paiements securises en crypto, garantit des transactions securisees.
Regarder de prГЁs|
ynaix – The aesthetic is clean and minimal, which works very well.
connectwiththeworldnow – Color scheme balanced, images and icons support the theme nicely.
Частный заем денег мфо домашние деньги альтернатива банковскому кредиту. Быстро, безопасно и без бюрократии. Получите нужную сумму наличными или на карту за считанные минуты.
strongrod – Found useful features and tools; impressed by utility.
wavefusion – Visual hierarchy is good, important stuff stands out immediately.
Все спортивные новости http://sportsat.ru в реальном времени. Итоги матчей, трансферы, рейтинги и обзоры. Следите за событиями мирового спорта и оставайтесь в курсе побед и рекордов!
brightideaweb – Overall nice polish, site feels professional and thoughtfully designed.
Sou louco pela vibracao de BR4Bet Casino, vibra como um farol em alto-mar. A colecao e um facho de diversao. com jogos adaptados para criptomoedas. Os agentes sao rapidos como um raio de farol. com solucoes precisas e instantaneas. Os saques voam como um facho de luz. de vez em quando queria mais promocoes que brilham como farois. Resumindo, BR4Bet Casino vale explorar esse cassino ja para os faroleiros do cassino! De bonus o design e fluido como uma lanterna. transformando cada aposta em uma aventura iluminada.
br4bet cassino|
metarise – Really enjoying the look, everything feels modern and smooth.
Sou louco pela torcida de MarjoSports Casino, explode com uma vibe de cassino esportiva. As opcoes sao ricas e vibram como torcidas. com caca-niqueis modernos que driblam como craques. O atendimento esta sempre ativo 24/7. respondendo veloz como um drible. O processo e claro e sem impedimentos. mas mais bonus seriam um diferencial de campo. Em resumo, MarjoSports Casino e uma rede de adrenalina para quem curte apostar com estilo esportivo! De lambuja a interface e fluida e vibra como um estadio. amplificando o jogo com vibracao de campo.
marjosports bonus de 10|
Estou completamente apaixonado por BETesporte Casino, oferece um prazer esportivo intenso. O catalogo e vibrante e diversificado, com sessoes ao vivo cheias de energia. Fortalece seu saldo inicial. O suporte ao cliente e excepcional, com suporte rapido e preciso. Os pagamentos sao seguros e fluidos, embora bonus mais variados seriam um golaco. No fim, BETesporte Casino oferece uma experiencia inesquecivel para amantes de apostas esportivas ! Vale destacar a navegacao e intuitiva e rapida, facilita uma imersao total. Um diferencial importante os torneios regulares para rivalidade, proporciona vantagens personalizadas.
Aprender como|
cyberlaunch – Content seems solid and meaningful, not just filler stuff.
Galera, resolvi contar como foi no 4PlayBet Casino porque foi muito alem do que imaginei. A variedade de jogos e de cair o queixo: slots modernos, todos rodando lisos. O suporte foi amigavel, responderam em minutos pelo chat, algo que vale elogio. Fiz saque em transferencia e o dinheiro entrou sem enrolacao, ponto fortissimo. Se tivesse que criticar, diria que seria legal torneios de slots, mas isso nao estraga a experiencia. Na minha visao, o 4PlayBet Casino me conquistou. Ja virou parte da minha rotina.
abaixar 4play bet baixar app|
sharpwave – Thoughtful layout, sections well separated and easy to scan.
Fiquei impressionado com PlayPIX Casino, oferece um prazer eletrizante. A selecao de jogos e fenomenal, incluindo apostas esportivas que aceleram o coracao. Eleva a experiencia de jogo. O suporte ao cliente e excepcional, acessivel a qualquer momento. Os ganhos chegam sem atraso, contudo ofertas mais generosas seriam bem-vindas. Em sintese, PlayPIX Casino e uma plataforma que brilha para amantes de emocoes fortes ! Acrescentando que o design e moderno e vibrante, adiciona um toque de conforto. Igualmente impressionante o programa VIP com niveis exclusivos, que aumenta o engajamento.
Aprender como|
brightchain – Design looks modern, I like the visual elements they chose.
innovatewithus – Content is super useful, I learned something new today.
cloudmark – Visuals are crisp and layout looks modern and polished.
bestchoiceonline – Checkout felt simple and secure, confidence inspiring.
metal band
nextbrand – The voice feels friendly and trustworthy, I like that.
digitalstorm – Pages load quickly, even complex graphics appear smooth.
everythingyouneedtoknow – Pages load fast, no waiting even on image-heavy content.
discoveramazingthingsonline – Really love the name, sounds exciting and adventurous.
explorecreativeideasdaily – Color palette is refreshing, helps keep me motivated while browsing.
ultrawave – The voice feels genuine, not trying too hard to impress.
yourbrandzone – Content is valuable, clearly written and well organized.
pixelplanet – Love the creativity here, visuals are really on point from first glance.
zenithmedia – Found good media resources, videos and articles both helpful.
boldspark – Typography is bold and readable, headings stand out clearly.
Suchen Sie Immobilien? http://www.montenegro-immobilien-kaufen.com/ wohnungen, Villen und Grundstucke mit Meerblick. Aktuelle Preise, Fotos, Auswahlhilfe und umfassende Transaktionsunterstutzung.
joinourcreativecommunity – Typography is friendly and readable, spacing makes it easygoing.
Je suis epate par Locowin Casino, ca procure un plaisir intense. La variete des titres est impressionnante, avec des slots au design innovant. Amplifiant l’experience initiale. Le support client est exceptionnel, garantissant un support de qualite. Les transactions sont fiables et rapides, neanmoins quelques tours gratuits en plus seraient cool. En bref, Locowin Casino est un incontournable pour les joueurs pour ceux qui aiment parier en crypto ! Notons aussi le site est rapide et attrayant, ce qui rend chaque session encore plus fun. Un autre atout majeur les paiements securises en crypto, assure des transactions fiables.
DГ©couvrir le site complet|
YourPathOfGrowth – The platform is user-friendly and full of helpful guides.
Je suis emerveille par Casinia Casino, il offre une aventure etincelante. Il y a une profusion de jeux envoutants, avec des slots aux designs audacieux et thematiques. 100% jusqu’a 500 € + 200 tours gratuits. Le service est disponible 24/7, toujours pret a eclairer. Les gains arrivent a la vitesse de la lumiere, de temps a autre des recompenses additionnelles seraient scintillantes. En bref, Casinia Casino garantit un plaisir radiant a chaque instant pour les aficionados de jeux modernes ! De plus l’interface est fluide comme un rayon, intensifie l’eclat du jeu. Egalement remarquable les tournois reguliers pour la rivalite, garantit des transactions fiables.
Commencer ici|
Je suis accro a Locowin Casino, ca procure un plaisir intense. Les options sont incroyablement vastes, offrant des sessions live dynamiques. Pour un demarrage en force. Le suivi client est irreprochable, offrant des reponses claires. Les paiements sont securises et fluides, parfois des offres plus genereuses ajouteraient du piquant. Pour conclure, Locowin Casino est un incontournable pour les joueurs pour les passionnes de jeux modernes ! De plus le design est moderne et fluide, ce qui rend chaque session encore plus fun. Egalement appreciable les evenements communautaires engageants, qui booste l’engagement.
Ouvrir le site maintenant|
LearnWithConfidence – Really satisfied, videos are concise and explanations feel super practical.
alphaimpact – Good contrast and color scheme; elements stand out clearly.
makingeverymomentcount – Articles are meaningful, tips seem practical and applicable right away.
thebestplacetostarttoday – The tone speaks to encouragement, not pressure, very refreshing.
learnshareandsucceed – Love the mission here, seems like a place to really grow together.
getreadytoexplore – The tone is curious and welcoming, makes me want to stay longer.
Estou completamente pixelado por PlayPix Casino, explode com uma vibe de cassino retro-futurista. Os jogos formam um render de diversao. com caca-niqueis que reluzem como bytes. Os agentes sao rapidos como um download. oferecendo respostas claras como um codigo. Os pagamentos sao seguros e fluidos. ocasionalmente mais bonus regulares seriam digitais. No fim das contas, PlayPix Casino oferece uma experiencia que e puro codigo para os viciados em emocoes de cassino! Por sinal o design e fluido como um render. criando uma experiencia de cassino cibernetica.
playpix cГіdigo de bГґnus|
everythingaboutsuccess – Overall very polished experience, feels like a premium motivational hub.
findwhatyouarelookingfor – Visuals support the message well, nothing feels out of place.
learnsomethingneweveryday – Love this idea, content always surprises me with new insights.
Estou alucinado com Fogo777 Casino, oferece uma aventura que reluz como brasas vivas. O leque do cassino e um fogo de delicias. oferecendo sessoes ao vivo que brilham como labaredas. Os agentes sao rapidos como uma faisca. assegurando apoio sem fumaca. As transacoes sao faceis como um fulgor. ocasionalmente mais bonus seriam um diferencial ardente. Resumindo, Fogo777 Casino vale explorar esse cassino ja para os amantes de cassinos online! Por sinal o layout e vibrante como uma tocha. elevando a imersao ao nivel de um ritual.
plataforma fogo777 Г© confiГЎvel|
discoverendlessinspiration – Love how much creativity this site sparks, feels like magic.
growyourbusinessfast – Found actionable tips right away, no filler content.
Estou completamente encantado com BETesporte Casino, oferece um prazer esportivo inigualavel. Ha uma explosao de jogos emocionantes, com slots modernos e tematicos. O bonus de boas-vindas e empolgante. O servico esta disponivel 24/7, oferecendo respostas claras. O processo e simples e direto, contudo promocoes mais frequentes dariam um toque extra. No fim, BETesporte Casino vale uma aposta certa para fas de cassino online ! Vale destacar o design e moderno e vibrante, instiga a prolongar o jogo. Notavel tambem o programa VIP com niveis exclusivos, assegura transacoes confiaveis.
Ir para o site|
makeimpactwithideas – Typography is crisp, spacing is comfortable for long reads.
Fiquei fascinado com PlayPIX Casino, proporciona uma aventura eletrizante. A selecao de jogos e fenomenal, incluindo apostas esportivas que aceleram o coracao. 100% ate €500 + rodadas gratis. Os agentes respondem com rapidez, com suporte rapido e preciso. As transacoes sao confiaveis, embora ofertas mais generosas seriam bem-vindas. No geral, PlayPIX Casino e uma plataforma que brilha para amantes de emocoes fortes ! Tambem o design e moderno e vibrante, adiciona um toque de conforto. Igualmente impressionante as opcoes variadas de apostas esportivas, assegura transacoes confiaveis.
Descobrir a web|
https://evarodriguez.fr/bonjour-tout-le-monde/
SimpleWaysToBeHappy – I find the daily affirmations helpful for a positive mindset.
BuildSomethingMeaningful – I appreciate how they focus on sustainable solutions for all.
GrowWithConfidenceHere – The community feedback is excellent, makes learning feel interactive and fun.
YourTrustedSourceOnline – I love how they integrate technology into their initiatives.
TogetherWeCreateChange – The community support is amazing, encourages everyone to share freely.
BuildABetterTomorrow – I appreciate how they focus on sustainable solutions for all.
CreateInspireAndGrow – This site has been a game-changer for my personal growth.
stayupdatedwithtrends – The tone is confident and helpful, not too buzzwordy.
thefutureofinnovation – Really loving this vision, innovation feels central and exciting here.
сеть фитнес клубов новый фитнес клуб
A vibrant platformer guiding a panda through exciting puzzles and treasures. Fun and family-friendly: new online games from Canada
Кейндин оюндары дайыма эмоция тартуулайт.Анын командалык иши да, жеке чеберчилиги да мыкты. Эгер Гарри Кейндин трансферлери кызыктырса, анда бул шилтемеге өт: [url=https://harry-kane-kg.com/]harry-kane-kg.com[/url]. Анын футбол стили классикалык жана эффективдүү.
Кейндин айлык акысы чоң талкууга себеп болду.
Анын үй-бүлөсү ар дайым бактылуу көрүнөт. Гол киргизүүдө ал өзгөчө инстинктке ээ. Футбол дүйнөсүндө Гарри Кейн өз ордун тапкан. Кейндин алгачкы командалары ага чоң тажрыйба берген. Ал ар бир сезон ондогон гол киргизет. Бул оюнчу тууралуу баары сүйлөшөт.
TurnIdeasIntoAction – The advice here is practical, not just theory, super useful.
EverythingStartsWithYou – This site reminds me: I’m in control of my path today.
Гарри Кейн ар дайым максатка умтулат.Анын командалык иши да, жеке чеберчилиги да мыкты. Кейндин күйөрмандары үчүн эң ишенимдүү булак — [url=https://harry-kane-kg.com/]Гарри Кейн жаңылыктары[/url]. Анын карьерасындагы ийгиликтер мактоого арзыйт.
Кейндин трансфери чоң көңүл бурдурган.
Кейн үй-бүлөлүк баалуулуктарды жогору коёт. Кейндин пас берүү жөндөмү да жогорку деңгээлде. Гарри Кейндин жашы чоң тажрыйбага жол ачат. Бул оюнчу ар дайым өнүгүүгө умтулган. Ал ар бир сезон ондогон гол киргизет. Анын келечеги жаркын көрүнөт.
LearnSomethingAwesome – I enjoy the mix of visuals and explanations, keeps me engaged.
FindTheBestIdeas – I enjoy the variety—some ideas are simple, others bold, mix keeps interest.
LearnShareAndGrow – This platform offers great resources for community development projects.
https://gobadam.com/does-rum-go-bad-expire-date-spoilage-signs/
TheArtOfLivingWell – Very clean layout, easy to navigate and find what I need.
FindNewWaysToGrow – Community stories add warmth and make the growth journey feel shared.
FindAnswersAndIdeas – This site gives practical answers, not just vague philosophy, which is rare.
фитнес клуб цены фитнес клуб с бассейном
YourDigitalDestination – I enjoy reading their posts, always learn something new.
Анын оюндары Англия футболунун сыймыгы.Гарри Кейндин PFA Premier League Team of the Year тизмесине киргени таң калычтуу эмес. Бул сайтта Кейндин толук статистикасы жана оюн жыйынтыктары бар: [url=https://harry-kane-kg.com/]Гарри Кейн статистикасы[/url]. Бул чабуулчу ар бир мелдеште гол киргизүүгө даяр.
Кейн менен Бавария дагы күчтүү болуп калды.
Кейн үй-бүлөлүк баалуулуктарды жогору коёт. Кейндин пас берүү жөндөмү да жогорку деңгээлде. Кейндин оюнун көрүү ырахат тартуулайт. Кейндин алгачкы командалары ага чоң тажрыйба берген. Гарри Кейндин оюндарынын саны укмуш. Анын Instagram баракчасы абдан популярдуу.
GetConnectedWithPeople – The notifications and messages are timely and don’t feel spammy.
UnlockYourPotentialToday – I like how ideas are broken into doable steps, super helpful.
FindSolutionsForLife – This site really helped me see problems through a fresh lens today.
ConnectDiscoverAndGrow – I’ve already saved a few articles to revisit when I need clarity.
DiscoverWorldOfIdeas – Definitely a resource I’ll revisit when I need creativity or new angles.
madeleinemtbc – I often share these updates with friends and neighbors
markmackenzieforcongress – Visiting this page gives me hope for better leadership and progress ahead.
FindInspirationEverywhere – I appreciate the mix of art, stories and practical advice here.
YourGuideForSuccess – Content is well paced, I don’t feel overwhelmed reading through lessons.
StartChangingYourLife – Every article feels like a small push forward for me.
DiscoverUsefulTipsDaily – The advice here is simple but surprisingly effective in practice.
DiscoverNewOpportunities – Their commitment to social impact is truly commendable.
BuildYourDreamFuture – I’m excited to try some of the strategies suggested here.
Кейндин оюндары дайыма эмоция тартуулайт.Анын командалык иши да, жеке чеберчилиги да мыкты. Футбол сүйүүчүлөрү бул шилтемеден пайдалуу маалымат ала алышат: [url=https://harry-kane-kg.com/]http://harry-kane-kg.com[/url]. Анын футбол стили классикалык жана эффективдүү.
Гарри Кейндин оюну ар дайым жогорку деңгээлде.
Кейндин үй-бүлөсү ар дайым анын жанында. Гол киргизүүдө ал өзгөчө инстинктке ээ. Анын физикалык даярдыгы мыкты. Ал бала чагынан эле футболчу болууну каалаган. Бул оюнчу кайсы чемпионатта болбосун айырмаланат. Анын Instagram баракчасы абдан популярдуу.
Кейндин карьерасы ар бир жаш оюнчуга үлгү.Футбол тарыхында анын аты чоң орунда болот. Гарри Кейн тууралуу так жана ишенимдүү маалымат алуу үчүн ушул булакты колдон: [url=https://harry-kane-kg.com/]Гарри Кейн маалымат[/url]. Бул чабуулчу ар бир мелдеште гол киргизүүгө даяр.
Кейндин трансфери чоң көңүл бурдурган.
Кейн үй-бүлөлүк баалуулуктарды жогору коёт. Гол киргизүүдө ал өзгөчө инстинктке ээ. Гарри Кейндин жашы чоң тажрыйбага жол ачат. Кейндин тарыхы ийгиликке ишенүүнүн далили. Кейндин статистикасын карасаң, анын чыныгы деңгээлин көрөсүң. Анын келечеги жаркын көрүнөт.
Откройте для себя прекрасные и загадочные места, которые находятся под охраной в нашей стране.
Между прочим, если вас интересует Изучение ООПТ России: парки, заповедники, водоемы, загляните сюда.
Вот, делюсь ссылкой:
[url=https://alloopt.ru]https://alloopt.ru[/url]
Спасибо за внимание! Надеюсь, вам было интересно.
Заказ автобуса на свадьбу https://povozkin.ru
bs2best at Неужели ты до сих пор не знаешь, что переворачивает мир даркнета? Blacksprut – это не просто название. Это новая эра анонимности, мгновенной скорости и железобетонной безопасности в сети. Загляни на bs2best.at – туда, где обсуждают то, о чём остальные предпочитают молчать в тряпочку. Тебе откроется доступ к информации, тщательно оберегаемой от посторонних глаз. Только для тех, кто понимает правила игры. Никаких следов, никаких компромиссов, только абсолютная конфиденциальность – это Blacksprut. Не упусти свой шанс стать первооткрывателем – bs2best.at уже распахнул свои двери для тебя. Хватит ли у тебя смелости взглянуть правде прямо в лицо?
Гарри Кейн оюн талаасында дайыма тынчсызданууну жаратат.Кейндин эмгеги жана дисциплинасы өзгөчө. Бул сайтта Гарри Кейндин Бавариядагы оюндары тууралуу кеңири маалымат бар: [url=https://harry-kane-kg.com/]Гарри Кейн Бавария</url]. Гарри Кейн дайыма өз командасына ишеним жаратат.
Кейндин трансфери чоң көңүл бурдурган.
Гарри Кейн жеке жашоосун ачык көрсөтпөйт. Кейндин карьерасы узак жана ийгиликтүү. Кейндин оюнун көрүү ырахат тартуулайт. Кейндин алгачкы командалары ага чоң тажрыйба берген. Гарри Кейн ар бир клубда өз изин калтырган. Анын келечеги жаркын көрүнөт.
Вас интересуют природные богатства России? Давайте исследуем их вместе!
Для тех, кто ищет информацию по теме “Изучение ООПТ России: парки, заповедники, водоемы”, нашел много полезного.
Ссылка ниже:
[url=https://alloopt.ru]https://alloopt.ru[/url]
Природа – наш главный учитель и защитник. Берегите её!
makethemostoflife – The content feels personal, not just generic advice slapped on
makeprogressdaily – This site has become a daily go-to for motivation.
Анын оюндары Англия футболунун сыймыгы.Ар бир сезон Гарри Кейн жаңы деңгээлге чыгат. Кейндин тарыхы жана жетишкендиктери боюнча кызыктуу макалаларды бул жерден тап: [url=https://harry-kane-kg.com/]Гарри Кейн биографиясы[/url]. Кейн Англиянын тарыхындагы эң жогорку бомбардирлердин бири.
Бавария күйөрмандары ага тез эле көнүштү.
Бул оюнчу үй-бүлөсүн биринчи орунга коёт. Кейн командалаштарын дайыма колдойт. Гарри Кейндин жашы чоң тажрыйбага жол ачат. Гарри Кейндин балалык сүрөттөрү интернетте көп. Ар бир күйөрман анын статистикасын сыймыктануу менен айтат. Бул оюнчу тууралуу баары сүйлөшөт.
Лазерные станки https://raymark.ru для резки металла в Москве. 20 лет на рынке, выгодная цена, скидка 5% при заявке с сайта + обучение
HOME CLIMAT https://homeclimat36.ru кондиционеры и сплит системы в Воронеже. Скидка на монтаж от 3000 рублей! При покупке сплит-системы.
inspireandgrowtogether – I appreciate the genuine tone, feels like real conversations.
findthebestcontent – Navigation is simple and gets me to what I want fast
findyourwayforward – The message gives hope and a clearer path, very nice
Добро пожаловать в удивительный мир природы России!
Особенно понравился материал про Изучение ООПТ России: парки, заповедники, водоемы.
Смотрите сами:
[url=https://alloopt.ru]https://alloopt.ru[/url]
Жду ваших отзывов и вопросов по теме.
discoverlearnandshare – Very educational content, feels like learning in fun way
growyourpresenceonline – I learned something I can use right away, appreciate it
licsupport – Helped me clear doubts about policy options, thank you
connectwithbrilliantminds – Very refreshing to see encouragement paired with clever ideas
everythingaboutmarketing – A must-visit site for staying updated on marketing best practices.
discoveryourpassion – Empowered me to pursue what truly lights me up.
discovertrendingideas – Easy to skim through and still catch strong ideas
Интернет платформы знакомств дают возможность взрослым людям общаться в удобном формате.
Такое знакомство расширяет кругозор.
Большинство людей отмечают, что современные платформы помогают отдохнуть после напряжённого дня.
Это удобный вариант для новых контактов.
https://cherylcole.ru/muzhchina-i-zhenshhina/porno-onlajn-inczest-interes-i-kulturnyj-kontekst/
Основное — сохранять вежливость и интерес в разговоре.
Лёгкое общение способствует настроение.
Такие площадки созданы для тех, кто хочет найти новых людей.
Современные сервисы становятся способом провести время с пользой.
learnandearndaily – It feels like someone wants to see readers succeed
connectandgrowonline – Highly recommend for anyone looking to enhance their online business.
jointhenextbigthing – The design is sleek, navigation is simple, very user-friendly.
learnexploreandshine – The voice is authentic, not overpolished or forced
togetherwecreateimpact – I appreciate how each post ties back to community betterment
findyourinspirationhere – Design is lovely, soft on the eyes and welcoming
Начнем путешествие по магическим уголкам российских заповедников.
По теме “Изучение ООПТ России: парки, заповедники, водоемы”, там просто кладезь информации.
Смотрите сами:
[url=https://alloopt.ru]https://alloopt.ru[/url]
Рад был поделиться с вами этой информацией. До новых встреч!
yourgatewaytosuccess – I was able to find resources fast, very smooth navigation
http://lukoshko.net/storyList/latyshskie-narodnye-skazki.htm
keepgrowingwithus – The content is uplifting and very well organized
Нужна недвижимость? https://www.nedvizhimost-chernogorii-u-morya.ru/ лучшие объекты для жизни и инвестиций. Виллы, квартиры и дома у моря. Помощь в подборе, оформлении и сопровождении сделки на всех этапах.
Аутстаффинг персонала https://skillstaff2.ru для бизнеса: легальное оформление сотрудников, снижение налоговой нагрузки и оптимизация расходов. Работаем с компаниями любого масштаба и отрасли.
Estou pirando com MegaPosta Casino, tem uma vibe de jogo que e pura dinamite. Tem uma enxurrada de jogos de cassino irados, com slots de cassino unicos e contagiantes. Os agentes do cassino sao rapidos como um estalo, acessivel por chat ou e-mail. Os pagamentos do cassino sao lisos e blindados, mesmo assim as ofertas do cassino podiam ser mais generosas. Em resumo, MegaPosta Casino vale demais explorar esse cassino para os aventureiros do cassino! Alem disso a navegacao do cassino e facil como brincadeira, aumenta a imersao no cassino a mil.
plataforma megaposta|
Estou completamente alucinado por OshCasino, e um cassino online que detona como um vulcao. Tem uma enxurrada de jogos de cassino irados, com caca-niqueis de cassino modernos e eletrizantes. O suporte do cassino ta sempre na ativa 24/7, garantindo suporte de cassino direto e sem cinzas. As transacoes do cassino sao simples como uma brisa, porem as ofertas do cassino podiam ser mais generosas. No geral, OshCasino e o point perfeito pros fas de cassino para os aventureiros do cassino! Alem disso o site do cassino e uma obra-prima de estilo, o que deixa cada sessao de cassino ainda mais incendiaria.
frais retrait osh|
Ich bin absolut hingerissen von NV Casino, es erzeugt eine Energie, die suchtig macht. Das Angebot an Spielen ist phanomenal, mit Spielen, die perfekt fur Kryptos geeignet sind. Der Support ist von herausragender Qualitat, mit praziser Unterstutzung. Die Auszahlungen sind ultraschnell, ab und zu zusatzliche Freispiele waren toll. Zum Abschluss, NV Casino bietet unvergesslichen Spa? fur Fans von Online-Wetten ! Au?erdem die Plattform ist optisch ein Highlight, was jede Session noch spannender macht.
https://playnvcasino.de/|
Sou viciado em BETesporte Casino, e uma plataforma que pulsa com emocao atletica. A variedade de titulos e impressionante, com slots modernos e tematicos. Com uma oferta inicial para impulsionar. O acompanhamento e impecavel, garantindo atendimento de alto nivel. Os ganhos chegam sem demora, embora bonus mais variados seriam um gol. Para finalizar, BETesporte Casino e uma plataforma que domina o campo para fas de cassino online ! Acrescentando que o design e moderno e dinamico, aumenta o prazer de apostar. Muito atrativo os pagamentos seguros em cripto, fortalece o senso de comunidade.
Aprender como|
UFCдеги рейтинги, рекорду жана статистикасы бир бетте чогултулуп, издөөнү жеңилдетет.
«Ислам Махачев кийинки ким менен?» деген суроого календарь жана промоутер маалыматтарынын негизинде жооп беребиз. [url=https://islam-makhachev-kg.com/]https://islam-makhachev-kg.com/[/url] Расмий профилдерге жана Sherdog барактарына туура жол көрсөтөбүз. Коопсуз шилтемелер гана колдонулат. Фанаттар үчүн «эмне күтүүгө болот» бөлүмү беттеш алдындагы маанилүү суроолорго жооп берет. Фанат пикирлери жана сурамжылоолор беттешке болгон кызыгууну көрсөтүп турат. Коучтар командасы жана лагерь логистикасы кыскача тизмектелет.
«Канча акча тапты?» сыяктуу суроолорго расмий булактарга таянып, болжолдуу сандар келтирилет. Коэффициенттер жана линиялар боюнча кыска гид: ким фаворит, ким андердог — объективдүү маалымат. Марапаттар, чемпиондук тизмек жана респект учурлары иллюстрация менен берилет. FAQ форматына ылайык, «качан беттеш болот», «кайдан көрөм», «канча салмакта ойнойт» деген суроолорго даяр жооптор бар.
UFCдеги рейтинги, рекорду жана статистикасы бир бетте чогултулуп, издөөнү жеңилдетет.
UFC 311 жакындап келе жатабы, Ислам Махачевтин расписаниеси реалдуу убакытта жаңыртылат. [url=https://islam-makhachev-kg.com/]ислам махачев рейтинг ufc[/url] Рейтингдеги абалы жана мүмкүн болгон титулдук беттештер тууралуу эксперттик пикирлер топтолгон. Объективдүү маалымат гана. Кимге каршы кандай план иштейт, кыска тактикалык эскертмелерди да кошобуз. Эгер ресми анонс чыкса, дата жана локация дароо жаңыланат. Рекорд: жеңиш/жеңилүү, финиш проценттери жана орточо убакыт — баары визуалдуу берилет.
«Канча акча тапты?» сыяктуу суроолорго расмий булактарга таянып, болжолдуу сандар келтирилет. Статистикага таянган прогноздор жана коопсуздук эскертмелери (жаңылыштык коркунучу) берилет. Сүрөттөр жана обои: социалдык тармак үчүн ылайыктуу форматтар жана резолюциялар бар. Издөө функциясы боюнча ачкыч сөздөрдү киргизип, керектүү бөлүмгө тез өтүңүз.
Строительный портал https://v-stroit.ru всё о строительстве, ремонте и архитектуре. Полезные советы, технологии, материалы, новости отрасли и практические инструкции для мастеров и новичков.
inspireeverymoment – Thank you for sharing these little sparks of inspiration.
shareyourvisiontoday – Great mix of inspiration and practical advice for visionaries.
votethurm – I saw this link on social media, looks like a political page.
growtogetherwithus – Love this community energy, feels like everyone’s learning together.
learnnewthingsdaily – This site really fuels my curiosity and passion to grow.
discovergreatideas – The content is thought-provoking and challenges conventional approaches.
thefuturestartsnow – Thanks for these reminders — feeling energized to move forward.
inspireeverymoment – This site makes me want to pause, reflect, act.
theartofsuccess – Love how the advice feels real and not overhyped at all.
startyourdigitaljourney – I love how user-friendly the interface is for beginners.
Карьерасынын бурулуш учурлары, мыкты раундар жана эмнелер аны чемпион кылганы тууралуу кеңири баяндалат.
Тикелей эфир башталганга чейин акыркы коэффициенттер жана линиялар жаңыртылып турат. [url=http://www.islam-makhachev-kg.com/]http://www.islam-makhachev-kg.com/[/url] Бул шилтеме аркылуу мобилдик версияга да бат өтөсүз. Жүктөлүү ылдамдыгы оптималдаштырылган. Жеткиликтүү интервьюлардан цитаталарды чогултуп, мотивация жана даярдык жөнүндө баяндайбыз. Илия Топурия vs Ислам Махачев айкашы жөнүндө ушак-айың эмес, текшерилген булактар гана берилет. Грепплинг жана клинчтеги көрсөткүчтөр статистикалык булактар менен ырасталат.
«Канча акча тапты?» сыяктуу суроолорго расмий булактарга таянып, болжолдуу сандар келтирилет. Хайптан алыстап, фактыларга таянган сабаттуу талкуу жүргүзөлү. Сүрөттөр жана обои: социалдык тармак үчүн ылайыктуу форматтар жана резолюциялар бар. Мобилдик түзмөктөр үчүн ылдам жүктөлүүчү версия жана ыңгайлуу меню жасалды.
yourjourneytowin – I bookmarked it, will revisit when I need to refocus.
createimpactfulstories – Such vivid storytelling, I feel connected and energized reading this.
staycuriousandcreative – Such a fresh perspective on blending curiosity with creativity.
Портал о строительстве https://pamel-stroy.ru и ремонте. Пошаговые инструкции, идеи, технологии, новости и советы экспертов. Всё, что нужно, чтобы строить и ремонтировать грамотно.
findyourcreativeflow – Thanks for reminding me creativity flows when I just let go.
Риэлторская контора https://daber27.ru покупка, продажа и аренда недвижимости. Помогаем оформить сделки безопасно и выгодно. Опытные риэлторы, консультации, сопровождение и проверка документов.
Купольные дома https://kupol-doma.ru под ключ — энергоэффективные, надёжные и современные. Проектирование, строительство и отделка. Уникальная архитектура, комфорт и долговечность в каждом доме.
Карьерасынын бурулуш учурлары, мыкты раундар жана эмнелер аны чемпион кылганы тууралуу кеңири баяндалат.
Телегид сыяктуу жөнөкөй форматта дата, убакыт жана оппоненттер тизилет. [url=https://islam-makhachev-kg.com/]ислам махачев рекорд[/url] Жеңиш/жеңилүү тарыхы жана финиш проценттери дайыма жаңыртылып турат. Диаграммалар кошулган. Алгачкы беттештеги негизги эпизоддорду эске салып, реванш үчүн сабактарды белгилеп беребиз. Эгер ресми анонс чыкса, дата жана локация дароо жаңыланат. Коучтар командасы жана лагерь логистикасы кыскача тизмектелет.
Акыркы беттештин толук обзорун раунд-раунд менен кыскача окуңуз. Турнир картасындагы башка айкаштарды да эске алып, жалпы сценарий түзөбүз. Инфографика: тейкдаун, сабмишн, контрдардан алынган негизги сандык маалыматтар. Максат — жаңжал эмес, так маалымат жана спорттук урмат.
Түз эфирге чейин негизги фактылар менен тааныштырып, беттештен соң жыйынтык талдоону чыгарабыз.
Картадагы ко-мэйн жана мэйн ивент боюнча да баалуу маалымдамалар кошулду. [url=https://islam-makhachev-kg.com/]ислам махачев рекорд[/url] Жеңиш/жеңилүү тарыхы жана финиш проценттери дайыма жаңыртылып турат. Диаграммалар кошулган. UFCдеги алардын позициялары, рекорддору жана окшош стилдери боюнча салыштырма таблица бар. Тарыхый коучинг линиясын, лагерь жаңылыктарын жана спарринг өнөктөштөрүн да карайбыз. Мурдагы атаандаштарынын стили менен салыштыруу аркылуу контекст берилет.
Ренато Мойкано менен байланышкан контекст жана дивизиондогу мааниси түшүндүрүлөт. Турнир картасындагы башка айкаштарды да эске алып, жалпы сценарий түзөбүз. Марапаттар, чемпиондук тизмек жана респект учурлары иллюстрация менен берилет. Жаңыртуулар тарыхы ачык көрсөтүлүп, өзгөртүүлөр датасы менен белгиленет.
https://jessehjjo292204.activoblog.com/44741474/treasures-of-the-sandswept-tombs
Ваша Недвижимость https://rbn-khv.ru сайт о покупке, продаже и аренде жилья. Разбираем сделки, налоги, ипотеку и инвестиции. Полезная информация для владельцев и покупателей недвижимости.
Информационный блог https://gidroekoproekt.ru для инженеров и проектировщиков. Всё об инженерных изысканиях, водохозяйственных объектах, гидротехническом строительстве и современных технологиях в отрасли.
Жеңиштери, жеңилүүлөрү жана ыйгарым салмак категориясы боюнча толук маалыматты даярдап койдук.
UFC 311 жакындап келе жатабы, Ислам Махачевтин расписаниеси реалдуу убакытта жаңыртылат. [url=http://www.islam-makhachev-kg.com/]http://www.islam-makhachev-kg.com/[/url] Бул шилтеме аркылуу мобилдик версияга да бат өтөсүз. Жүктөлүү ылдамдыгы оптималдаштырылган. Алгачкы беттештеги негизги эпизоддорду эске салып, реванш үчүн сабактарды белгилеп беребиз. Тарыхый коучинг линиясын, лагерь жаңылыктарын жана спарринг өнөктөштөрүн да карайбыз. Беттешке чейинки форма, спарринг жана медициналык текшерүүлөр боюнча расмий шилтемелер бар.
«Ислам Махачев кимге утулду?» деген суроого тарыхый тизмек жана шарттар менен жооп беребиз. Жаракат тууралуу ушак-айыңдан сактануу үчүн текшерилген жаңылыктар гана колдонулат. Тренинг залынан фрагменттер жана күрөш ыкмалары тууралуу түшүндүрмөлөр кошулат. Издөө функциясы боюнча ачкыч сөздөрдү киргизип, керектүү бөлүмгө тез өтүңүз.
mystylecorner – Browsing was fun, discovered pieces I didn’t even know I needed.
creativevision – The abstract photography here is truly captivating and thought-provoking.
filamericansforracialaction.com – Good to see WordPress used, content seems accessible and organized.
stacoa.org – I’ll bookmark this and come back to read more.
myvetcoach.org – Excited to see what new posts you’ll put out next.
Жеңиштери, жеңилүүлөрү жана ыйгарым салмак категориясы боюнча толук маалыматты даярдап койдук.
Пресс-конференция жана тарсылдатуу жыйынтык видеолору бет ачылгандан кийин эле жеткиликтүү. [url=https://islam-makhachev-kg.com/]ислам махачев рейтинг ufc[/url] Рейтингдеги абалы жана мүмкүн болгон титулдук беттештер тууралуу эксперттик пикирлер топтолгон. Объективдүү маалымат гана. Жеткиликтүү интервьюлардан цитаталарды чогултуп, мотивация жана даярдык жөнүндө баяндайбыз. Мүмкүн болгон апсеттер жана беттеш темпи боюнча аналитикалык бөлүм бар. Беттешке чейинки форма, спарринг жана медициналык текшерүүлөр боюнча расмий шилтемелер бар.
Түз эфирден кийинки статустар, бонустар жана трофейлер тууралуу фактылар кошулат. Коэффициенттер жана линиялар боюнча кыска гид: ким фаворит, ким андердог — объективдүү маалымат. Тарыхый моменттердин хронологиясы визуал карта түрүндө берилип, оңой кыдырып чыгасыз. Жаңылыктарга жазылсаңыз, ири анонстар чыккан замат билдирүү аласыз.
electlarryarata – The design is simple, would love to see your platform soon.
thespeakeasybuffalo – The site is promising, just needs more substance now.
laetly.com – Couldn’t see much content, looking forward to updates.
norigamihq – I tried loading it but didn’t see much content yet.
newseason – The layout is modern, design feels balanced and inviting.
successpartnersgroup – I always recommend this site to friends who appreciate quality.
trendyfashioncorner – Great variety of options, from casual to chic looks.
growyourdigitalpresence – Articles feel honest, not just hype — I appreciate that.
summerstageinharlem.org – Great community vibe, bringing local music and culture together.
globalideasnetwork – Their mission and projects are impressive, site supports that nicely.
pinellasehe.org – Great writing style, engaging without being overwhelming.
rockyrose.org – The colors and layout make browsing a delightful experience.
cryptotephra-clagr.com – If you want, I can check whether this site is actively maintained and reachable.
discovernewideas – I like how everything is laid out, clean and creative vibes.
createimpact – Got inspired by the content, got me thinking of new possibilities.
hecimagine.com – I hope they feature student stories and application details soon.
inspiredgrowthteam – Got a good first impression, looks like they put effort in.
formative-coffee.com – Really feel the passion behind every detail on this site.
ukrainianvictoryisthebestaward.com – Looks like the domain’s been reused or repurposed.
luxurytrendstore – Checkout process was quick and hassle-free, very user-friendly.
bs2web at Приготовься узнать, что взорвало тёмные глубины интернета. Blacksprut – это не просто бренд. Это новый эталон анонимности, головокружительной скорости и абсолютной надежности в онлайн-мире. Посети bs2best.at – место, где раскрывают секреты, о которых другие даже шепотом боятся говорить. Ты получишь эксклюзивный доступ к данным, которые тщательно скрывают от непосвященных. Только для тех, кто в теме и готов к большему. Ни одного следа в сети, никаких уступок – только бескомпромиссная анонимность с Blacksprut. Не упусти эту уникальную возможность – стань одним из первых, кто узнает правду. bs2best.at уже ждёт тебя. Готов ли ты увидеть то, что скрыто от большинства?
visionaryleadersclub – Good branding, the look matches the promise they make.
Estou pirando total com JabiBet Casino, e um cassino online que e uma verdadeira onda. A gama do cassino e simplesmente um maremoto, com caca-niqueis de cassino modernos e envolventes. O atendimento ao cliente do cassino e uma mare de qualidade, respondendo mais rapido que um maremoto. Os ganhos do cassino chegam voando como uma onda, mas mais recompensas no cassino seriam um diferencial brabo. Na real, JabiBet Casino oferece uma experiencia de cassino que e puro fluxo para os cacadores de slots modernos de cassino! Alem disso o design do cassino e uma explosao visual aquatica, aumenta a imersao no cassino como uma onda gigante.
jabibet|
Acho simplesmente brabissimo PagolBet Casino, da uma energia de cassino que e um raio. O catalogo de jogos do cassino e uma explosao total, oferecendo sessoes de cassino ao vivo que sao um relampago. O servico do cassino e confiavel e brabo, dando solucoes na hora e com precisao. Os pagamentos do cassino sao lisos e blindados, de vez em quando mais recompensas no cassino seriam um diferencial brabo. No geral, PagolBet Casino vale demais explorar esse cassino para os amantes de cassinos online! E mais o design do cassino e uma explosao visual vibrante, da um toque de voltagem braba ao cassino.
pagolbet.|
Ich bin fasziniert von SpinBetter Casino, es ist eine Erfahrung, die wie ein Wirbelsturm pulsiert. Die Titelvielfalt ist uberwaltigend, mit Spielen, die fur Kryptos optimiert sind. Die Hilfe ist effizient und pro, verfugbar rund um die Uhr. Der Ablauf ist unkompliziert, obwohl regelma?igere Aktionen waren toll. Global gesehen, SpinBetter Casino ist eine Plattform, die uberzeugt fur Online-Wetten-Fans ! Hinzu kommt die Site ist schnell und stylish, verstarkt die Immersion. Ein weiterer Vorteil die mobilen Apps, die den Einstieg erleichtern.
spinbettercasino.de|
partnershippowerhouse – Having clean design plus meaningful content makes all the difference here.
Ich habe eine Leidenschaft fur NV Casino, es ist ein Abenteuer, das pulsiert wie ein Herzschlag. Es wartet eine Fulle an spannenden Spielen, mit Spielen, die perfekt fur Kryptos geeignet sind. Der Kundensupport ist hervorragend, erreichbar zu jeder Stunde. Die Transaktionen sind zuverlassig, dennoch regelma?igere Promos waren super. Zum Abschluss, NV Casino ist definitiv empfehlenswert fur Fans von Online-Wetten ! Hinzu die Oberflache ist intuitiv und stylish, fugt eine Prise Magie hinzu.
playnvcasino.de|
Tenho uma paixao vibrante por BETesporte Casino, proporciona uma aventura eletrizante. As opcoes sao amplas como um campo de futebol, com sessoes ao vivo cheias de energia. Com uma oferta inicial para impulsionar. O suporte ao cliente e excepcional, sempre pronto para entrar em campo. Os saques sao rapidos como um contra-ataque, contudo mais apostas gratis seriam incriveis. Em sintese, BETesporte Casino oferece uma experiencia inesquecivel para amantes de apostas esportivas ! Acrescentando que a plataforma e visualmente impactante, aumenta o prazer de apostar. Notavel tambem as opcoes variadas de apostas esportivas, que impulsiona o engajamento.
https://betesporte365.app/|
discovernewpath – I’d share this with friends, seems like something worth knowing.
findyourfuture – Found useful info quickly, layout feels very friendly.
nextvision – Wish there was proof of legitimacy—user reviews, company registration etc.
uniquefashionstyle – The minimalist design aligns perfectly with the brand’s aesthetic.
http://www.Vegabetyardim.com ile Vegabet’te yaşayabileceğiniz tüm problemleri çözüyoruz. Vegabet’e giriş yaparak sorunsuz bahis keyfine devam edebilirsiniz.
trustconnectionnetwork – The site’s aesthetic is modern and appealing, reflecting quality.
Современные онлайн-сервисы для обработки изображений считаются всё более востребованными.
Они применяют AI-технологии для обновления изображений.
С помощью таких ботов можно удалить фон на фото без опыта.
Это экономит время и обеспечивает высокое качество.
раздеватор тг
Современные пользователи выбирают такие боты для портфолио.
Они способствуют создавать эстетичные фотографии даже в домашних условиях.
Интерфейс таких систем простое, поэтому с ними легко разобраться.
Развитие нейросетей превращает фотообработку эффективной для всех пользователей.
trustedbusinesslink – Definitely a place I’d recommend for professional business connections.
futureopportunitynetwork – A valuable tool for anyone seeking future opportunities.
Register at casino glory and receive bonuses on your first deposit on online casino games and slots right now!
growstrongteam – I’d recommend this site to anyone seeking positive mindset resources.
https://pikman.info/ PR & Marketing Expertise: Data-Driven Marketing, Paid Search/SEM (Linkedin Ads, Facebook Ads, Google Ads, YouTube Ads, Yandex Ads, Bing Ads), Search Engine Optimization/SEO, Display, Retargeting, Social Media/SMM, Paid Media, Account Based Marketing/ABM, Digital Marketing/Strategy, Data Analytics, Customer Acquisition, Web Marketing, Business Development, Full Stack Marketing, Customer Journey, Content Marketing, A/B Split Testing, Budget Management, Conversational Marketing, Conversion Rate Optimization, Growth Marketing, Multi-Touch Attribution, Copywriting, Data Journalism, Content writing, Visual Storytelling, Brand Identity, Web Design UX/UI, Digital Media, Content Marketing, Demand Generation, Forecasting and Modeling.
futurevisiongroup – Definitely a site I’d revisit — lots of promise here.
learnsharegrow – Really like how they mix learning with sharing, feels community-oriented.
Vegabet’in canlı bahis seçeneği, kullanıcıların maçları izlerken heyecanlarını katlayarak bahis yapmalarını sağlar. Kullanıcılar, maçın gidişatına göre tahminlerde bulunabilir ve anlık olarak bahislerini yapabilirler. Bu sayede, kullanıcılar maçın heyecanını daha da artırırken aynı zamanda kazanç elde etme şansına sahip olurlar. http://www.vegabetadres.com
happylifestylehub – Everything here feels authentic and uplifting, thumbs up.
growthpoint – Seems legit, good presentation, minimal noise and clutter.
futureopportunitynetwork – Good balance between branding, content, and user experience.
futuremindscollective – Looking forward to more collections that inspire and educate.
Деревянные лестницы https://rosslestnica.ru под заказ в любом стиле. Прямые, винтовые, маршевые конструкции из массива. Замеры, 3D-проект, доставка и установка. Гарантия качества и точности исполнения.
Лестницы в Москве https://лестницы-в-москве.рф продажа и изготовление под заказ. Прямые, винтовые, модульные и чердачные конструкции. Качество, гарантия и монтаж по всем стандартам.
teamfuture – It feels like a space for growth, not just passive reading.
businessgrowthhub – The design is polished, makes learning about business enjoyable.
trustandunity – The visuals support the message, creating a cohesive feel.
digitalinnovationzone – Learning something new every visit, keeps me hooked.
futuregoalsnetwork – Always nice to see substance behind style, this delivers.
https://plisio.net/th/blog/sell-cbd-with-crypto-plisio-s-ecwid-plugin
successbridge – I’ve bookmarked this site, think it’ll be valuable long-term.
getthedeal – Recommended for anyone, site is reliable and updated often enough.
https://www.bestomegawatches.com/so-beautiful-omega-seamaster-aqua-terra-jewellery-replica-watch-for-2019/162-2/
leadersunitedgroup – Clear layout, easy to find what you need, no clutter.
connectinnovationhub – Content is helpful and practical, not just theory — much appreciated.
winmore – I like the energy here, feels ambitious and motivating.
findsomethingnew – Content here is fresh and vibrant, keeps me coming back.
futuregoalsnetwork – Always excited to check updates — this site has potential.
trustconnectionnetwork – Always find something meaningful, good balance of inspiration and info.
successjourneyclub – Great balance between inspiration and actionable steps, love that.
discovervalue – Every article gives me something to think about and apply.
globalpartnershipteam – Always nice to see realism and value mixed together well here.
discoverdaily – Always something to learn, the content is solid and helpful.
leadersunitedgroup – The visuals support the writing nicely, feels cohesive.
video show
exploreideasworld – I’ve bookmarked it — here’s where ideas get real substance.
connectinnovationhub – Great site for connecting ideas and people — truly inspiring.
growtogetheralliance – Clean design, easy navigation — makes browsing a calm experience.
brightdeal – Already bookmarked this — feels like long-term potential exists.
innovateandbuild – The design’s clean and modern, makes exploring fun and easy.
buildtogether – I really love the community feel here, so welcoming.
growthnetwork – I feel like this site could become a trusted resource soon.
Milanobet, müşteri hizmetleri konusunda da başarılı bir performans sergilemektedir. Kullanıcıların herhangi bir sorunla karşılaşması durumunda, 7/24 hizmet veren canlı destek hattı sayesinde sorunlarına anında çözüm bulabilmektedirler. Ayrıca, Milanobet’in sunduğu bonus ve promosyonlar da kullanıcıları cezbetmektedir. Yüksek oranlarla sunulan bonuslar, kullanıcıların kazançlarını artırmalarına yardımcı olmaktadır. http://www.milanobet.net
Sou louco pela energia de BRCasino, oferece uma aventura de cassino que pulsa como um pandeiro. A gama do cassino e simplesmente um sambodromo de delicias, incluindo jogos de mesa de cassino com um toque de carnaval. Os agentes do cassino sao rapidos como um mestre-sala, com uma ajuda que brilha como serpentinas. O processo do cassino e limpo e sem tropecos, porem queria mais promocoes de cassino que botam pra quebrar. Em resumo, BRCasino oferece uma experiencia de cassino que e puro batuque para os folioes do cassino! De lambuja a interface do cassino e fluida e reluz como uma fantasia de carnaval, eleva a imersao no cassino ao ritmo de um tamborim.
br77 slot login|
Sou louco pelo batuque de RioPlay Casino, oferece uma aventura de cassino que pulsa como um tamborim. A gama do cassino e simplesmente um bloco de carnaval, com slots de cassino tematicos de festa. A equipe do cassino entrega um atendimento que e puro carnaval, acessivel por chat ou e-mail. Os ganhos do cassino chegam voando como um mestre-sala, porem mais recompensas no cassino seriam um diferencial brabo. Na real, RioPlay Casino oferece uma experiencia de cassino que e puro axe para os folioes do cassino! De bonus o design do cassino e um desfile visual colorido, adiciona um toque de axe ao cassino.
cassino rioplay|
Galera, preciso compartilhar minha experiencia no 4PlayBet Casino porque superou minhas expectativas. A variedade de jogos e bem acima da media: jogos ao vivo imersivos, todos bem otimizados ate no celular. O suporte foi eficiente, responderam em minutos pelo chat, algo que faz diferenca. Fiz saque em Bitcoin e o dinheiro entrou sem enrolacao, ponto fortissimo. Se tivesse que criticar, diria que mais brindes fariam falta, mas isso nao estraga a experiencia. No geral, o 4PlayBet Casino e parada obrigatoria pra quem gosta de cassino. Vale experimentar.
three minutes 4play|
Fiquei impressionado com PlayPIX Casino, e uma plataforma que pulsa com energia festiva. As opcoes sao vastas como um desfile, com sessoes ao vivo dinamicas. Fortalece seu saldo inicial. O servico esta disponivel 24/7, sempre pronto para ajudar. Os pagamentos sao seguros e fluidos, embora bonus mais variados seriam bem-vindos. Para concluir, PlayPIX Casino e uma plataforma que reina suprema para quem aposta com cripto ! Vale notar o design e moderno e vibrante, instiga a prolongar a experiencia. Muito atrativo os eventos comunitarios envolventes, proporciona vantagens personalizadas.
Ler os detalhes|
Tenho uma paixao intensa por BETesporte Casino, transporta para um mundo de apostas vibrante. O catalogo e rico e diversificado, com sessoes ao vivo imersivas. Fortalece seu saldo inicial. O acompanhamento e impecavel, acessivel a qualquer hora. Os pagamentos sao seguros e fluidos, de vez em quando ofertas mais generosas dariam um toque especial. Para finalizar, BETesporte Casino oferece uma experiencia inesquecivel para amantes de apostas esportivas ! Adicionalmente a interface e fluida e energetica, aumenta o prazer de apostar. Um diferencial importante o programa VIP com niveis exclusivos, oferece recompensas continuas.
Descobrir agora mesmo|
businessgrowthhub – Clean design makes learning easy, I’m enjoying exploring.
blsp at Приготовься узнать, что взорвало тёмные глубины интернета. Blacksprut – это не просто бренд. Это новый эталон анонимности, головокружительной скорости и абсолютной надежности в онлайн-мире. Посети bs2best.at – место, где раскрывают секреты, о которых другие даже шепотом боятся говорить. Ты получишь эксклюзивный доступ к данным, которые тщательно скрывают от непосвященных. Только для тех, кто в теме и готов к большему. Ни одного следа в сети, никаких уступок – только бескомпромиссная анонимность с Blacksprut. Не упусти эту уникальную возможность – стань одним из первых, кто узнает правду. bs2best.at уже ждёт тебя. Готов ли ты увидеть то, что скрыто от большинства?
Skill move practice efficiency improved after choosing to buy fifa coins for five-star skillers instead of grinding with clunky players lacking technical abilities. Real active sellers with fast delivery and non drop guarantees enable gameplay improvement through proper player selection matching your mechanical skill development goals.
Ищу красивые обои 4K с Неймаром: барса, ПСЖ или ранний «Сантос» — что посоветуете для телефона? Где скачать удобный apk, чтобы получать пушки о голах Неймара прямо во время canlı izləmə? Для мобил фанатов mobil futbol важна чистая сводка по матчам и травмам. [url=https://neymar-kg.com/]неймар статистика[/url]. Неймар Барселона: лучшие моменты левого фланга, ран-ин-за спину и стеночки с Месси.
Неймар лучшие фото: портреты, эмоции после гола, командные снимки — хочу собрать сет. Неймар барса — обои с Камп Ноу и ночной подсветкой стадиона смотрятся мощно. Неймар в детстве: редкие фото из академии «Сантоса» — подскажите архивы. Где играет Неймар 2025 — обновляю карточки игрока в своём блоге.
Неймар барса обои 4К — важно проверить соотношение сторон под ультраширокие мониторы. Неймар возраст и рост/вес.
https://plisio.net/it/blog/jay-z-net-worth
smartpartnership.bond – It’s nice to see collaboration being highlighted rather than just profit.
smartdesigncorner.shop – Found inspiration for my next project, thanks for sharing.
Слив курсов ЕГЭ русский 2025 https://courses-ege.ru
partnershippowerhouse.shop – Layout is clean, easy to navigate without feeling overwhelming.
globalunity.bond – Already thinking how I might collaborate or connect here.
trustinyou.bond – Definitely sharing this with friends who might find this message helpful.
inspiredmind.shop – Love the creative vibe here, so many fresh inspiring items.
shopwithpurpose.shop – I bookmarked several things — love the mindful style here.
smartdesigncorner.space – Very creative touch in colors and layout, really engaging.
Ищу красивые обои 4K с Неймаром: барса, ПСЖ или ранний «Сантос» — что посоветуете для телефона? Сравнение Неймара 2011 и 2017: темп, решения, пауза — отличный кейс для тактического разбора. Нужны «неймар обои 4к» без водяных знаков и с правильным соотношением сторон. [url=https://neymar-kg.com/]неймар обои 4к[/url]. Нужны «обои неймар 4к» без компрессии и с правильным кропом под разные экраны.
Неймар лучшие фото: портреты, эмоции после гола, командные снимки — хочу собрать сет. Неймар заработная плата: интересно увидеть разрез по контрактам и бонусам, если есть открытые данные. Неймар жена, дети — только официальные факты, без слухов; подскажите корректные био-источники. Неймар барса — лучшие фото с трофеями и парадами, для коллекции.
Неймар обой — встречал такое написание: ищу корректные теги для поиска. Неймар новости — лучше краткие дайджесты, чем длинные простыни текста.
creativegiftzone.shop – Such a fresh selection, makes gift shopping fun instead of stressful.
creativityuniverse.shop – This could be a goldmine for gifting ideas and creative inspiration.
buildbrand.online – Their case studies page alone motivates me to rethink my own brand.
trendfinder.bond – The layout feels modern and everything loads really fast too.
inspiredgrowthteam.shop – The tone is uplifting, makes me feel like growth is possible.
inspiredideas.shop – Found some ideas I hadn’t thought of — thanks for this.
dailyvaluehub.shop – Great variety, competitive prices, and quality looks promising too.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
startsomethingnew.shop – Great design, smooth navigation — makes exploring a joy.
buildlastingtrust.bond – Trust-building content feels honest, not just flashy marketing.
findnewtrend.shop – I bookmarked a few items; will revisit later.
https://clocks-top.com/
trustandunity.shop – Sharing this with friends — feels like something worth knowing about.
brightfuturegroup.bond – Bookmarking this, want to follow their updates over time.
smartstylehub.shop – Easy navigation, categories are clean and well organized.
https://plisio.net/el/blog/jasmycoin-jasmy-price-prediction
Кто знает, сколько голов забил Неймар за карьеру? Хочу таблицу по клубам и сборной. Интересует «неймар рост вес возраст» по годам — удобнее видеть в одной инфографике. Хочу таблицу голов за карьеру и распределение по клубам/сборной. [url=https://neymar-kg.com/]сколько голов забил неймар[/url]. Где играет Неймар 2024/2025 и как выглядит его роль? Интересно для анализа комбинаций.
Ищу «неймар барселона фото» с классическими комбинациями с Суаресом и Месси. Где играет Неймар сейчас — обновляю подписи к постам, чтобы не ошибаться. Сравнение прайма Неймара в «Барселоне» и пик-сезонов в ПСЖ — таблица метрик. Неймар барса — лучшие фото с трофеями и парадами, для коллекции.
Где найти «неймар сантос фото» в HQ, не из соцсетей с пережатием? Неймар новости — лучше краткие дайджесты, чем длинные простыни текста.
https://plisio.net/el/blog/onlyfans-stock
globalpartnershipteam.bond – Love the global vision here, feels inclusive and ambitious.
modernchoice.store – I like how clean the design is, space makes browsing relaxing.
trustedbusinesslink.shop – Seems like they care about trust and clarity, good sign.
buildlastingties.bond – The site gives me a vibe of real community bonding.
visionaryleadersclub.bond – Love seeing leadership content that’s practical and engaging.
music connect
Советы по выбору обоев Неймара: матчи, портреты, training — что лучше для экрана смартфона? Нужна подборка «месси роналду неймар фото» для заставки — редкие кадры и матчевые эмоции приветствуются. Нужен проверенный источник без лишней рекламы — только факты и фото. [url=www.neymar-kg.com]www.neymar-kg.com[/url]. Сантос Неймар: финты против плотной обороны — полезно разобрать для молодых вингеров.
Неймар Бразилия: обои в национальной форме, стадионный свет и динамика движения. Сравниваю «неймар 2012 сантос» и переход в Европу — интересна динамика решений в финале. Неймар жена, дети — только официальные факты, без слухов; подскажите корректные био-источники. Неймар барса — лучшие фото с трофеями и парадами, для коллекции.
Неймар фото ПСЖ — лучше матч-день, где эмоции максимально живые. Неймар возраст и рост/вес.
Неймар в «Барселоне» и ПСЖ: где был пик дриблинга и ассистов, по цифрам и визуальному анализу? Где скачать удобный apk, чтобы получать пушки о голах Неймара прямо во время canlı izləmə? Полезные советы по выбору обоев и актуальный номер игрока в клубе. [url=https://neymar-kg.com/]где играет неймар[/url]. Где играет Неймар 2024/2025 и как выглядит его роль? Интересно для анализа комбинаций.
Нужны «неймар обои на телефон» с мягким контрастом, чтобы иконки читались. Нужны «обои на телефон футбол Неймар» в вертикальном формате 1080×2400. Неймар лысый — мемные кадры смешные, но ищу оригиналы без копипасты. Неймар барса — лучшие фото с трофеями и парадами, для коллекции.
Неймар какой клуб сейчас — нужен снэпшот для карточек игроков. Неймар возраст и рост/вес.
https://plisio.net/it/blog/krea-ai
Me enredei no algoritmo de PlayPix Casino, e um cassino online que reluz como um pixel em overclock. Os jogos formam um render de diversao. incluindo jogos de mesa com um toque cibernetico. O atendimento e solido como um pixel. respondendo veloz como um byte. Os pagamentos sao lisos como um buffer. entretanto mais giros gratis seriam uma loucura cibernetica. Para encurtar, PlayPix Casino oferece uma experiencia que e puro codigo para os viciados em emocoes de cassino! Vale dizer a plataforma reluz com um visual glitch. dando vontade de voltar como um byte.
playpix Г© de qual pais|
Adoro o gingado de SambaSlots Casino, da um ritmo de cassino que e puro axe. O catalogo de jogos do cassino e uma folia total, com slots de cassino tematicos de festa. A equipe do cassino entrega um atendimento que e puro carnaval, respondendo mais rapido que um tamborim. As transacoes do cassino sao simples como um passo de samba, de vez em quando as ofertas do cassino podiam ser mais generosas. No fim das contas, SambaSlots Casino garante uma diversao de cassino que e uma folia total para os viciados em emocoes de cassino! De lambuja a plataforma do cassino brilha com um visual que e puro ritmo, torna a experiencia de cassino uma festa inesquecivel.
casino polygone sambaslots cagnes sur mer|
Ich liebe den Wahnsinn von JackpotPiraten Casino, es bietet ein Casino-Abenteuer, das wie ein Schatz funkelt. Es gibt eine Flut an packenden Casino-Titeln, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Die Casino-Mitarbeiter sind schnell wie ein Piratenschiff, liefert klare und schnelle Losungen. Casino-Gewinne kommen wie ein Blitz, manchmal wurde ich mir mehr Casino-Promos wunschen, die funkeln. Kurz gesagt ist JackpotPiraten Casino ein Casino, das man nicht verpassen darf fur Spieler, die auf krasse Casino-Kicks stehen! Ubrigens die Casino-Navigation ist kinderleicht wie ein Kartenlesen, was jede Casino-Session noch aufregender macht.
jackpotpiraten casino|
investsmarttoday – Clean layout and fast loading, makes exploring investment tips enjoyable.
Je trouve absolument fantastique 7BitCasino, ca procure une sensation de casino unique. Le catalogue est incroyablement vaste, avec des machines a sous modernes et captivantes. Le service d’assistance est de premier ordre, offrant des reponses rapides et precises. Les retraits sont ultra-rapides, neanmoins les promotions pourraient etre plus genereuses, ou des promotions hebdomadaires plus frequentes. Pour conclure, 7BitCasino est un incontournable pour les joueurs en quete d’adrenaline ! Ajoutons que le site est concu avec style et modernite, facilite chaque session de jeu.
7bitcasino opiniones|
successjourneyclub.shop – Already thinking about checking their resources and courses.
brightmarketplace – Fast shipping and excellent customer service, highly recommend shopping here.
classytrendstore – Smooth browsing and secure payment options, very trustworthy site.
smartbuytoday – Impressed with the packaging and timely delivery, will shop again.
bestdealcorner – Found unique items not available elsewhere, great shopping experience.
Fiquei fascinado com BETesporte Casino, oferece um prazer esportivo intenso. A selecao de jogos e fenomenal, oferecendo jogos de mesa envolventes. Com uma oferta inicial para impulsionar. Os agentes respondem com rapidez, oferecendo respostas claras. Os ganhos chegam sem atraso, as vezes mais apostas gratis seriam incriveis. Resumindo, BETesporte Casino vale uma aposta certa para fas de cassino online ! Tambem a plataforma e visualmente impactante, aumenta o prazer de apostar. Muito atrativo o programa VIP com niveis exclusivos, fortalece o senso de comunidade.
Saiba mais|
justvotenoon2 – Really practical advice, I’ll definitely try these suggestions soon.
Ищу красивые обои 4K с Неймаром: барса, ПСЖ или ранний «Сантос» — что посоветуете для телефона? Интересует «неймар рост вес возраст» по годам — удобнее видеть в одной инфографике. Собираю редкие «неймар сантос фото» в высоком качестве — делюсь находкой. [url=neymar-kg com]neymar-kg com[/url]. Где играет Неймар 2024/2025 и как выглядит его роль? Интересно для анализа комбинаций.
Ищу «неймар барселона фото» с классическими комбинациями с Суаресом и Месси. Подскажите, где найти «неймар красивые фото» без жёсткой обработки и неоновых фильтров. Неймар в детстве: редкие фото из академии «Сантоса» — подскажите архивы. Неймар ПСЖ 2018 — топовый сезон по влиянию между линиями; ищу срез по матчам.
Неймар фото ПСЖ — лучше матч-день, где эмоции максимально живые. Где сейчас играет Неймар — закреплю ссылку в профиле, чтобы не терять.
dailyprofitupdate – The site loads fast; navigation is intuitive and user-friendly.
modernvisionlab.shop – The photos are nice quality, helps in deciding if to buy.
stylemebetter – Browsing here was smooth; product descriptions are clear and helpful.
everydayvaluefinds.shop – The way values are presented feels genuine, not just sales pitch.
Ищу красивые обои 4K с Неймаром: барса, ПСЖ или ранний «Сантос» — что посоветуете для телефона? Неймар номер: менялся ли он в «Сантосе», «Барселоне», ПСЖ и «Аль-Хиляль» — нужен список по сезонам. Нужен факт-чек по слухам и корректные био-детали без кликбейта. [url=https://neymar-kg.com/]неймар 2025[/url]. Неймар в «Сантосе» 2011 — редкие хайлайты находить всё сложнее; делитесь проверенными плейлистами.
Неймар рисунок: ищу референсы для скетчей — профиль, движение, финт с остановкой. Неймар религия: редкая тема, важно корректно и без спекуляций — есть проверенные источники? Неймар завершил карьеру? Хочу факт-чек от надёжных спортивных СМИ. Неймар фото 2024 — без пересвета, контраст средний, чтобы текст поверх был виден.
Неймар какая зарплата в «Аль-Хиляль» — интересует структура фикс+бонусы. Неймар новости — лучше краткие дайджесты, чем длинные простыни текста.
modernvaluecorner – Great value for money; products match the descriptions perfectly.
findyourlook.shop – Definitely sharing this with friends who love fashion and trends.
buildlastingtrust.shop – Their messaging feels genuine, not just trying to sell something.
nextgenproject.shop – I appreciate how bold the name is — strong branding.
trendhunterplace – Excellent design and fast loading, overall great experience today.
yourtradingmentor – Helpful insights for traders, both beginners and experienced will benefit greatly.
trendinggiftcorner – Loved the gift ideas here, very creative and fun selections.
smarttradingmentor – Helpful insights and strategies, learned a lot just browsing the tutorials.
modernwoodcases – The quality of the materials used is impressive and durable.
purebeautyoutlet – Great product variety here, descriptions are clear and helpful.
telecharger 1xbet apk telecharger 1xbet cameroun
musionet – Useful resources for beginners and advanced users alike, thanks.
protraderacademy – Useful strategies and practical advice, saves a lot of time learning.
tradingmasterclass – Helpful guides and resources, learned a lot in just a few minutes.
strongpartnershipnetwork – Loved the advice and insights, makes networking much easier to manage.
newhorizonsnetwork.bond – Definitely feels like they’re trying to build something meaningful.
Новости спорта онлайн http://sportsat.ru футбол, хоккей, бокс, теннис, баскетбол и другие виды спорта. Результаты матчей, обзоры, интервью, аналитика и главные события дня в мире спорта.
fastgrowthsignal – Great value for price, shipping details transparent and delivery reliable.
ultimateprofitplan – Loved the actionable insights, really helped streamline my operations.
https://b2shop.gl
marketanalysiszone – Thoughtful interpretations, will definitely revisit before making decisions.
betting bonus promotions Boost your bankroll with lucrative betting welcome bonuses. Start with a bigger balance and enhance your potential winnings. Join now!
learnandtrade – Bookmarking this site, will revisit for more trading tips soon.
teamworksuccesspath – Found useful strategies for building effective teamwork and achieving goals together.
classyhomegoods – This store is now on my go-to list for stylish home décor shopping.
successnetworkgroup – Site is clean, navigation worked smoothly and found good resources quickly.
forexstrategyguide – Well-structured content, made complex concepts easy to understand.
shopwithsmile – Fast website load and straightforward shopping experience overall.
globalchoicehub – The customer service responded quickly when I had a question.
shopandshine – Customer support responded quickly and helped me sort my query.
visionpartnersclub – Loved the product selection, quality items at reasonable prices here.
ultimateprofitplan – Clear and concise, perfect for entrepreneurs looking to grow efficiently.
yourtradingmentor – Helpful resources and tools that feel relevant to real market conditions.
bestforexstrategies – Very interesting site, found some fresh ideas for trading I’ll try soon.
successfultradersclub – I like that they emphasize risk management alongside trade setups — very smart.
nextleveltrading – Found some useful tools here, very impressed with the content quality.
futuregrowthteam – Loved the product selection, quality items at reasonable prices here.
shoplocaltrend – Fast shipping and excellent customer service, highly recommend shopping here.
strongpartnershipnetwork – Helpful content and easy navigation, keeps me coming back regularly for tips.
strongfoundation – Helpful content, practical suggestions that seem applicable immediately.
everydaytrendshop – Stylish fashion finds, loved the unique designs and quality.
uniquedecorstore – The website is easy to browse and items look high quality.
simplelivinghub – Love the clean aesthetic, useful tips for a more peaceful home.
globalmarketinsight – Felt trustworthy and professional, important for market research resources.
classyhomegoods – Trendy styles at great prices, found exactly what I needed.
smartbuytoday – Loved discovering new items I didn’t know I needed, very fun experience.
trendandstyle – Smooth browsing and secure payment options, very trustworthy site.
learnandtrade – This plan is a game-changer for anyone serious about business growth.
https://www.vidxden.com/
freshstylemarket – Loved the packaging, items arrived in perfect condition.
Все о коттеджных посёлках https://cottagecommunity.ru фото, описание, стоимость участков и домов. Всё о покупке, строительстве и жизни за городом в одном месте. Полезная информация для покупателей и инвесторов.
Ich liebe die Energie von PlayJango Casino, es ist ein Online-Casino, das wie ein Wirbelsturm tobt. Es gibt eine Woge an packenden Casino-Titeln, mit modernen Casino-Slots, die einen in ihren Bann ziehen. Der Casino-Support ist rund um die Uhr verfugbar, ist per Chat oder E-Mail erreichbar. Casino-Gewinne kommen wie ein Komet, dennoch die Casino-Angebote konnten gro?zugiger sein. Am Ende ist PlayJango Casino ein Casino, das man nicht verpassen darf fur Abenteurer im Casino! Ubrigens die Casino-Navigation ist kinderleicht wie ein Windhauch, den Spielspa? im Casino in die Hohe treibt.
playjango casino no deposit|
Estou completamente alucinado por SpeiCasino, oferece uma aventura de cassino que orbita como um satelite. A selecao de titulos do cassino e um buraco negro de emocoes, oferecendo sessoes de cassino ao vivo que brilham como nebulosas. O servico do cassino e confiavel e brilha como uma galaxia, dando solucoes na hora e com precisao. Os ganhos do cassino chegam voando como um meteoro, mesmo assim mais giros gratis no cassino seria uma loucura estelar. No fim das contas, SpeiCasino e o point perfeito pros fas de cassino para os apaixonados por slots modernos de cassino! Vale dizer tambem a plataforma do cassino brilha com um visual que e puro cosmos, eleva a imersao no cassino a um nivel cosmico.
spei monthly bonus|
Sou louco pela ressonancia de JonBet Casino, pulsa com uma forca de cassino digna de um sino. Tem uma enxurrada de jogos de cassino irados. com caca-niqueis que vibram como harpas. O servico e confiavel como uma camara. oferecendo respostas claras como uma nota. Os pagamentos sao lisos como uma corda. de vez em quando mais giros gratis seriam uma loucura sonora. Resumindo, JonBet Casino e um cassino online que e uma camara de diversao para os maestros do cassino! Vale dizer o design e um espetaculo visual harmonico. criando uma experiencia de cassino harmonica.
como usar o bonus da jonbet|
Estou completamente apaixonado por BacanaPlay Casino, da uma energia de cassino que e pura purpurina. As opcoes de jogo no cassino sao ricas e cheias de gingado, com jogos de cassino perfeitos pra criptomoedas. Os agentes do cassino sao rapidos como um passista na avenida, respondendo mais rapido que um batuque de pandeiro. Os saques no cassino sao velozes como um carro alegorico, mas queria mais promocoes de cassino que botam pra quebrar. No geral, BacanaPlay Casino e o point perfeito pros fas de cassino para os folioes do cassino! De lambuja a plataforma do cassino brilha com um visual que e puro samba, faz voce querer voltar ao cassino como num desfile sem fim.
bacanaplay bonuskode|
bs2best зеркало Приготовься узнать, что взорвало тёмные глубины интернета. Blacksprut – это не просто бренд. Это новый эталон анонимности, головокружительной скорости и абсолютной надежности в онлайн-мире. Посети bs2best.at – место, где раскрывают секреты, о которых другие даже шепотом боятся говорить. Ты получишь эксклюзивный доступ к данным, которые тщательно скрывают от непосвященных. Только для тех, кто в теме и готов к большему. Ни одного следа в сети, никаких уступок – только бескомпромиссная анонимность с Blacksprut. Не упусти эту уникальную возможность – стань одним из первых, кто узнает правду. bs2best.at уже ждёт тебя. Готов ли ты увидеть то, что скрыто от большинства?
Tenho um entusiasmo vibrante por PlayPIX Casino, sinto um pulsar selvagem. As opcoes sao amplas como uma selva, com sessoes ao vivo cheias de energia. Eleva a experiencia de jogo. O servico esta disponivel 24/7, acessivel a qualquer momento. O processo e simples e elegante, no entanto promocoes mais frequentes dariam um toque extra. Em sintese, PlayPIX Casino oferece uma experiencia memoravel para jogadores em busca de adrenalina ! Alem disso a interface e fluida e estilosa, aumenta o prazer de jogar. Muito atrativo os torneios regulares para competicao, assegura transacoes confiaveis.
Encontrar os detalhes|
musionet – The site loads fast and the sections are well-structured for users.
urbanstylehub – Customer service was responsive when I had a query, appreciated that.
dailytrendstore – Found quality products at decent prices, happy with the selection.
winwithus.bond – I appreciate the variety, keeps discovering new useful ideas.
growthnetworkgroup.cfd – The platform’s design is intuitive, making networking seamless and efficient.
trendandstyle – Trendy styles at great prices, found exactly what I needed.
financialgrowthplan – Found helpful resources for budgeting and growth, quite impressed.
justvotenoon2 – Website loads fast, nice layout and easy to navigate sections.
brightmarketplace – Packaging was neat, items arrived in perfect condition — very satisfied.
broadcast chat
successfultradersclub – Will bookmark this site, it looks like a solid resource to revisit later.
modernwoodcases – Quality felt really solid when I held the sample, seems built to last.
businesssuccesshub – Will bookmark this site—it’s becoming a go-to for growth insights.
tradingmasterclass – Excellent platform for learning advanced trading techniques in detail.
rasecurities – This site will definitely go into my bookmarks for future reference.
tradeandwin – Found a tip I hadn’t seen elsewhere, useful little gem.
markettrendalerts – Found a signal I hadn’t noticed before, very helpful for decision-making.
fitproawardsuk – This has become a go-to landmark for anyone aiming to shine in the fitness profession.
Нужна вывеска? неоновая вывеска спб логотипы, надписи, декор для кафе, офисов и дома. Индивидуальный дизайн, энергосберегающие материалы и эффектный свет в любом стиле.
https://bs2tsite1.at
strongpartnershipnetwork.cfd – A trustworthy hub for connection and growth among professional networks today.
Нужен сайт? создание сайтов под ключ с акцентом на конверсию: UX-исследование, дизайн-система, чистая вёрстка, CMS на выбор, подключение метрик и событий. Интернет-магазины, B2B-порталы, лендинги. SEO-структура, микроразметка, скорость 90+. Поддержка и развитие без скрытых расходов.
Все о коттеджных посёлках https://cottagecommunity.ru как выбрать локацию, проверить инфраструктуру и коммуникации, понять цены и налоги. Сравнение ИЖС/ДНП, надёжность застройщиков, ипотека и субсидии, отзывы жителей, карта проектов и чек-листы для осмотра. Поможем принять взвешенное решение о покупке.
Ich finde absolut majestatisch Richard Casino, es verstromt eine Spielstimmung, die wie ein Kronjuwel glanzt. Die Auswahl im Casino ist ein wahres Kronungsjuwel, mit Casino-Spielen, die fur Kryptowahrungen optimiert sind. Das Casino-Team bietet Unterstutzung, die wie ein Zepter glanzt, liefert klare und schnelle Losungen. Der Casino-Prozess ist klar und ohne Intrigen, manchmal mehr regelma?ige Casino-Boni waren furstlich. Zusammengefasst ist Richard Casino ein Casino mit einem Spielspa?, der wie ein Thron funkelt fur Fans moderner Casino-Slots! Und au?erdem die Casino-Seite ist ein grafisches Meisterwerk, Lust macht, immer wieder ins Casino zuruckzukehren.
why is richard branson in casino royale|
Строительство и ремонт https://repair-house.kiev.ua дома без ошибок: пошаговые инструкции, выбор материалов и подрядчиков, смета и экономия, фундамент, кровля, инженерия, утепление, отделка. Чек-листы, калькуляторы, типовые узлы, лайфхаки по срокам и качеству. Экономим бюджет — повышаем комфорт.
Ich bin suchtig nach King Billy Casino, es bietet ein Casino-Abenteuer, das wie ein Kronungsfest funkelt. Der Katalog des Casinos ist eine Schatzkammer voller Spa?, mit modernen Casino-Slots, die verzaubern. Der Casino-Kundenservice ist wie ein koniglicher Hof, sorgt fur sofortigen Casino-Support, der beeindruckt. Der Casino-Prozess ist klar und ohne Intrigen, trotzdem die Casino-Angebote konnten gro?zugiger sein. Insgesamt ist King Billy Casino eine Casino-Erfahrung, die wie ein Palast glitzert fur Fans moderner Casino-Slots! Extra das Casino-Design ist ein optisches Kronungsjuwel, den Spielspa? im Casino auf ein konigliches Niveau hebt.
king billy casino en ligne|
Estou completamente enfeiticado por SpellWin Casino, tem uma vibe de jogo tao fascinante quanto um grimorio antigo. A gama do cassino e simplesmente um feitico de prazeres, com slots de cassino tematicos de fantasia. A equipe do cassino entrega um atendimento que e puro encantamento, respondendo mais rapido que um estalo magico. Os ganhos do cassino chegam voando como uma vassoura encantada, porem mais giros gratis no cassino seria uma loucura magica. Resumindo, SpellWin Casino e o point perfeito pros fas de cassino para os amantes de cassinos online! De lambuja o site do cassino e uma obra-prima de estilo mistico, adiciona um toque de encantamento ao cassino.
spellwin germany|
Acho simplesmente estelar BetorSpin Casino, e um cassino online que gira como um asteroide em chamas. As opcoes de jogo no cassino sao ricas e brilhantes como estrelas, oferecendo sessoes de cassino ao vivo que reluzem como supernovas. O atendimento ao cliente do cassino e uma estrela-guia, com uma ajuda que reluz como uma aurora boreal. Os saques no cassino sao velozes como uma viagem interestelar, de vez em quando as ofertas do cassino podiam ser mais generosas. No geral, BetorSpin Casino e o point perfeito pros fas de cassino para os apaixonados por slots modernos de cassino! Alem disso o design do cassino e um espetaculo visual intergalactico, o que torna cada sessao de cassino ainda mais estelar.
betorspin sports betting|
Estou completamente apaixonado por PlayPIX Casino, e uma plataforma que transborda dinamismo. Ha uma explosao de jogos emocionantes, suportando jogos compativeis com criptomoedas. Fortalece seu saldo inicial. O acompanhamento e impecavel, acessivel a qualquer momento. O processo e simples e elegante, de vez em quando recompensas extras seriam eletrizantes. Em sintese, PlayPIX Casino oferece uma experiencia memoravel para entusiastas de jogos modernos ! Adicionalmente a plataforma e visualmente espetacular, adiciona um toque de conforto. Outro destaque os torneios regulares para competicao, que aumenta o engajamento.
Ver agora|
Upgrade your anonymity game with an antidetect browser. Designed for large agencies and advanced users, it offers robust features for automation, team collaboration, and high-level digital stealth.
Bahis sitelerinin çevrim şartları genellikle belirli bir miktar bahis yapılmasını ve belirli oranlarda bahislerin gerçekleştirilmesini içerebilir. Örneğin, bonusun belirli bir katına kadar bahis yapma şartı veya minimum oranlarda bahis yapma zorunluluğu gibi kurallar sıkça karşılaşılan şartlar arasındadır. Bu şartlara uygun olarak bahis yapmak, bonusun kazanca dönüşmesi için önemlidir.
bs market Заинтригован, что вызывает резонанс в тёмных закоулках сети? Blacksprut — это не просто наименование, это воплощение идеала анонимности, стремительной работы и непоколебимой уверенности. Отправляйся на bs2best.at — в обитель, где раскрывают секреты, о которых предпочитают молчать. Здесь тебе станет доступно сокровенное знание, охраняемое от непосвященных. Исключительно для тех, кто понимает суть. Никаких улик. Никаких соглашений. Только Blacksprut. Не упусти драгоценную возможность стать пионером — bs2best.at распахнул врата для тех, кто ищет истину. Хватит ли тебе отваги заглянуть правде в лицо?
Портал о строительстве домов https://doma-land.ru проекты и сметы, сравнение технологий (каркас, газобетон, кирпич, брус), фундамент и кровля, инженерия и утепление. Калькуляторы, чек-листы, тендер подрядчиков, рейтинги бригад, карта цен по регионам, готовые ведомости материалов и практика без ошибок.
Vegabet’in güncel promosyonları için telegram adresini takip etmeyi unutmayın. https://t.me/VegaResmi
Квартира с отделкой https://новостройкивспб.рф экономия времени и предсказуемый бюджет. Фильтруем по планировкам, материалам, классу дома и акустике. Проверяем стандарт отделки, толщину стяжки, ровность стен, работу дверей/окон, скрытые коммуникации. Приёмка по дефект-листу, штрафы за просрочку.
Estou completamente empolgado com BETesporte Casino, oferece um thrill esportivo unico. O catalogo e rico e diversificado, com slots modernos e tematicos. Eleva a experiencia de jogo. Os agentes respondem com velocidade, garantindo atendimento de alto nivel. As transacoes sao confiaveis, as vezes bonus mais variados seriam um gol. No fim, BETesporte Casino vale uma aposta certa para jogadores em busca de emocao ! Adicionalmente a navegacao e intuitiva e rapida, tornando cada sessao mais competitiva. Muito atrativo os eventos comunitarios envolventes, oferece recompensas continuas.
Descobrir mais|
Estou completamente vidrado por SupremaBet Casino, parece um reinado de diversao avassaladora. A gama do cassino e simplesmente uma dinastia de delicias, incluindo jogos de mesa de cassino com um toque de realeza. O suporte do cassino ta sempre na ativa 24/7, acessivel por chat ou e-mail. As transacoes do cassino sao simples como um mandato, de vez em quando mais bonus regulares no cassino seria supremo. No geral, SupremaBet Casino e um cassino online que e um reino de diversao para os soberanos do cassino! Vale dizer tambem a navegacao do cassino e facil como um decreto real, o que torna cada sessao de cassino ainda mais majestosa.
supremabet|
Ich bin abhangig von SpinBetter Casino, es liefert ein Abenteuer voller Energie. Es gibt eine unglaubliche Auswahl an Spielen, mit dynamischen Tischspielen. Die Agenten sind blitzschnell, garantiert top Hilfe. Die Transaktionen sind verlasslich, dennoch mehr abwechslungsreiche Boni waren super. Zum Ende, SpinBetter Casino ist absolut empfehlenswert fur Online-Wetten-Fans ! Daruber hinaus die Plattform ist visuell ein Hit, erleichtert die gesamte Erfahrung. Zusatzlich zu beachten die schnellen Einzahlungen, die Vertrauen schaffen.
https://spinbettercasino.de/|
Estou alucinado com DiceBet Casino, e um cassino online que e puro fogo. O catalogo de jogos do cassino e uma loucura total, incluindo jogos de mesa de cassino cheios de classe. Os agentes do cassino sao rapidos como um foguete, respondendo mais rapido que um raio. O processo do cassino e direto e sem treta, de vez em quando as ofertas do cassino podiam ser mais generosas. No fim das contas, DiceBet Casino oferece uma experiencia de cassino que e puro gas para quem curte apostar com estilo no cassino! De lambuja o design do cassino e uma explosao visual, o que deixa cada sessao de cassino ainda mais animal.
dicebet casino|
чому треба берегти природу
Красивые девушки с нежными руками делают массаж незабываемым. Каждое движение продумано и мягкое, ощущаешь заботу и внимание. Атмосфера салона располагает к полному расслаблению и гармонии. Очень советую, индивидуалка недорого нск – https://sibirka.com/. Обязательно вернусь снова, всё очень понравилось.
SEO-продвижение позволяет компании увеличивать видимость в интернете и привлекать целевых клиентов. Оптимизация сайта включает работу с контентом, структурой страниц и техническими параметрами. Регулярный мониторинг и аналитика помогают выявлять слабые места и корректировать стратегию. В результате ресурс становится удобным и надёжным для пользователей. Такой подход обеспечивает устойчивый рост бизнеса. Читайте подробнее https://harry.main.jp/mediawiki/index.php/Сео_Продвижение_Сайтов_Для_Малого_Бизнеса
Продвижение сайта помогает бизнесу развивать онлайн-присутствие и увеличивать количество заказов. Оптимизация включает работу с контентом, внутренними ссылками и техническими настройками. Регулярный анализ конкурентов и корректировка стратегии поддерживают позиции и обеспечивают стабильный результат. Сайт становится более удобным для пользователей и привлекает больше клиентов. Такой подход подходит для долгосрочного развития компании. Подробнее по ссылке https://docs.brdocsdigitais.com/index.php/User:WalkerBleau199
Fiquei impressionado com PlayPIX Casino, transmite uma energia vibrante. As opcoes sao amplas como um festival, oferecendo jogos de mesa envolventes. Eleva a experiencia de jogo. A assistencia e eficiente e amigavel, oferecendo respostas claras. Os ganhos chegam sem atraso, as vezes ofertas mais generosas seriam bem-vindas. Para finalizar, PlayPIX Casino e uma plataforma que brilha para jogadores em busca de adrenalina ! Acrescentando que o site e rapido e cativante, adiciona um toque de conforto. Outro destaque os pagamentos seguros em cripto, proporciona vantagens personalizadas.
Descobrir mais|
Продвижение сайта — это комплексный процесс, направленный на привлечение целевой аудитории. SEO улучшает позиции ресурса, делает сайт удобным и информативным. Оптимизация включает работу с контентом, метатегами и техническими параметрами. Такой подход обеспечивает стабильный поток клиентов и долгосрочный эффект. Подробнее о SEO-продвижении читайте – https://itformula.ca/index.php?title=Эффективное_Сео_Продвижение_Сайтов_Любой_Тематики
Компания «СибЗТА» https://sibzta.su производит задвижки, клапаны и другую трубопроводную арматуру с 2014 года. Материалы: сталь, чугун, нержавейка. Прочные уплотнения, стандарты ГОСТ, индивидуальные решения под заказ, быстрая доставка и гарантия.
Ich bin vollig verzaubert von Trickz Casino, es ist ein Online-Casino, das wie ein Zauberstab funkelt. Die Auswahl im Casino ist ein echtes Hexenwerk, inklusive stilvoller Casino-Tischspiele. Der Casino-Kundenservice ist wie ein magischer Assistent, antwortet blitzschnell wie ein magischer Funke. Der Casino-Prozess ist klar und ohne Tauschung, ab und zu mehr Freispiele im Casino waren ein magischer Volltreffer. Alles in allem ist Trickz Casino eine Casino-Erfahrung, die wie ein Zaubertrick glanzt fur die, die mit Stil im Casino wetten! Extra die Casino-Navigation ist kinderleicht wie ein Zauberspruch, was jede Casino-Session noch verzauberter macht.
trickz casino flashback|
Ich bin vollig geflasht von Lapalingo Casino, es pulsiert mit einer elektrisierenden Casino-Energie. Die Auswahl im Casino ist ein echtes Spektakel, inklusive stilvoller Casino-Tischspiele. Der Casino-Service ist zuverlassig und glanzend, liefert klare und schnelle Losungen. Auszahlungen im Casino sind schnell wie ein Wirbelwind, dennoch wurde ich mir mehr Casino-Promos wunschen, die explodieren. Insgesamt ist Lapalingo Casino ein Casino mit einem Spielspa?, der wie ein Feuerwerk knallt fur Fans von Online-Casinos! Zusatzlich die Casino-Oberflache ist flussig und strahlt wie ein Nordlicht, den Spielspa? im Casino in den Himmel hebt.
lapalingo 100 bonus|
Ich schatze sehr Billy Billion Casino, es bietet ein Eintauchen in eine pulsierende Spielwelt. Der Katalog ist unglaublich umfangreich, mit progressiven Jackpots wie Mega Moolah. Der Support ist blitzschnell uber Live-Chat, mit hervorragendem Follow-up. Gewinne kommen in Rekordzeit an, jedoch zusatzliche VIP-Belohnungen waren willkommen. Kurz gesagt ist Billy Billion Casino eine au?ergewohnliche Plattform fur Fans von Nervenkitzel! Nicht zu vergessen ist die Benutzeroberflache flussig und modern mit einem Cartoon-Design, die Immersion verstarkt.
billy billion casino 33 free spins|
Поиск фирмы для строительства — ответственный момент при реализации ремонта.
Прежде чем заключить договор, стоит оценить опыт компании.
Компетентная фирма всегда гарантирует качественные услуги.
Стоит учесть, какие методы входят в работу при возведении объектов.
https://hackernoon.com/preview/y9k8LmYYyzSEqKrfiYFS
Just desire to say your article is as astounding. The clarity in your post is simply great and i can assume you’re an expert on this subject. Fine with your permission allow me to grab your feed to keep up to date with forthcoming post. Thanks a million and please keep up the enjoyable work.
https://staffinggoals.com/
купить мухомор Магазин в сети muhomorus предоставляет возможность заказать мухоморы с доставкой по всей России. У нас выгодные цены на экологически чистые продукты, предназначенные для борьбы с беспокойством, стрессом, упадком сил, постоянной усталостью и ослабления признаков различных недугов. Высушенные мухоморы не числятся лекарственным средством, а причисляются к парафармацевтической продукции, являющейся нетрадиционным методом, используемым индивидуально в качестве дополнительного лечения. Сбор, высушивание, реализация и приобретение – полностью законны. Поэтому, мы предлагаем вам приобрести микродозинг совершенно легально.
Отличный сервис, который всегда выручает в нужный момент. DRINKIO доставляет быстро, аккуратно и без лишних звонков. Курьеры пунктуальные, работают вежливо и спокойно. Ассортимент впечатляет, всё оригинальное. Радует, что доставка работает круглосуточно и без сбоев. Прекрасное решение для заказа алкоголя на дом по Москве https://drinkio105.ru/
Thank you a bunch for sharing this with all folks you really know what you’re speaking approximately! Bookmarked. Please also talk over with my site =). We will have a hyperlink trade arrangement between us
easystaffingmd.com
J’adore la chaleur de VBet Casino, c’est un casino en ligne qui jaillit comme un volcan en furie. Les options de jeu au casino sont riches et enflammees, comprenant des jeux de casino adaptes aux cryptomonnaies. Les agents du casino sont rapides comme une etincelle, joignable par chat ou email. Les retraits au casino sont rapides comme une coulee de lave, parfois les offres du casino pourraient etre plus genereuses. Pour resumer, VBet Casino est une pepite pour les fans de casino pour les amoureux des slots modernes de casino ! Par ailleurs l’interface du casino est fluide et eclatante comme un cratere en fusion, ce qui rend chaque session de casino encore plus enflammee.
mail vbet|
Ich bin total begeistert von Lowen Play Casino, es bietet ein Casino-Abenteuer, das wie ein Urwald leuchtet. Der Katalog des Casinos ist ein Dschungel voller Nervenkitzel, mit Live-Casino-Sessions, die wie ein Sturm donnern. Das Casino-Team bietet Unterstutzung, die wie ein Konig regiert, liefert klare und schnelle Losungen. Casino-Zahlungen sind sicher und reibungslos, trotzdem mehr Freispiele im Casino waren ein wilder Triumph. Zusammengefasst ist Lowen Play Casino ein Casino, das man nicht verpassen darf fur Abenteurer im Casino! Ubrigens die Casino-Plattform hat einen Look, der wie eine Savanne funkelt, einen Hauch von Abenteuer ins Casino bringt.
löwen play mitarbeiter angebote|
Ich bin abhangig von SpinBetter Casino, es erzeugt eine Spielenergie, die fesselt. Es gibt eine unglaubliche Auswahl an Spielen, mit immersiven Live-Sessions. Die Agenten sind blitzschnell, verfugbar rund um die Uhr. Die Transaktionen sind verlasslich, gelegentlich zusatzliche Freispiele waren ein Highlight. Global gesehen, SpinBetter Casino ist ein Muss fur alle Gamer fur Online-Wetten-Fans ! Zusatzlich die Navigation ist kinderleicht, was jede Session noch besser macht. Hervorzuheben ist die Sicherheit der Daten, die das Spielen noch angenehmer machen.
spinbettercasino.de|
Galera, nao podia deixar de comentar sobre o Bingoemcasa porque me ganhou de verdade. O site tem um ambiente divertido que lembra um encontro descontraido. As salas de bingo sao sempre lotadas, e ainda testei a roleta ao vivo, todos me deixaram impressionado. O atendimento no chat foi rapido como nunca vi, o que ja me deixou tranquilo. As retiradas foram muito rapidas, inclusive testei cartao e foi impecavel. Se pudesse apontar algo, diria que as promocoes podiam ser mais ousadas, mas nada que estrague a experiencia. Na minha visao, o Bingoemcasa vale demais a pena. Vale testar sem medo
bingoemcasa codigo promocional|
Collaboration with influencers has become one of the most popular strategies in online promotion.
It allows companies to build relationships with their audience through the voice of influential people.
Influencers produce content that boost engagement in a brand.
The main advantage of this approach is its genuine communication.
https://rafaelecxn38495.timeblog.net/69304985/how-social-media-stars-are-transforming-consumer-behavior-through-authentic-campaigns
Users tend to react more actively to personal stories than to traditional advertising.
Marketers can effectively select influencers to attract the right audience.
A thought-out influencer marketing campaign enhances visibility.
As a result, this method of promotion has become an essential part of digital communication.
Качественная замена лобового стекла не только восстанавливает обзор и безопасность, но и гарантирует корректную работу датчиков и систем автомобиля, таких как ассистенты движения и автоматический дворники. Доверяя работу профессионалам, вы обеспечиваете долговечность и надежность результата: замена лобового стекла
Mobil futbol izləyərkən paralel mərci tək kliklə yerləşdirmək olur — interfeys sadə və sürətlidir.
Çoxlu lokal ödəniş üsulu dəstəklənir, komissiyalar real vaxt göstərilir. Yeni başlayanlar qeydiyyatdan sonra [url=https://pinco-casino-azerbaijan.biz.ua/]pinco casino az[/url] təkliflərini yoxlaya və demo rejimdə oyunlarla tanış ola bilər. Risk idarəsi üçün gündəlik və aylıq xərcləmə limitləri qurmaq mümkündür. Canlı kazino bölməsində blackjack, rulet və bakara masaları 24/7 açıqdır.
Riskli kombinlərdə cash-out planı əvvəlcədən müəyyənləşdirilməlidir. Məlumat sərfiyyatını azaltmaq üçün axın keyfiyyətini ayarlardan endirmək olar.
Dəstək 24/7 işləyir, chat cavabları adətən 1–2 dəqiqəyə gəlir. Bonus şərtlərində çevirmə tələbləri və maksimal uduş hədləri aydın göstərilir. Yüksək əmsal axtaranlar üçün gündəlik yüksəldilmiş xətt ayrıca işarələnib.
Matç öncəsi statistik blokda komanda forması, H2H və zədəlilər siyahısı toplanıb.
Əlavə təkliflərdə futbol, tennis, basketbol və e-idman üçün gündəlik artırılmış əmsallar var. Aktiv promosları qaçırmamaq üçün [url=https://pinco-casino-azerbaijan.biz.ua/]pinco bonus[/url] bölməsinə baxın, canlı izləmə zamanı əlavə fribetlər çıxır. Komissiya və limit cədvəlləri müştəri razılaşmasında açıq yazılıb. Minimum və maksimum mərc diapazonları həm yeni başlayanlar, həm də high-rollerlər üçün uyğundur.
Canlı mərclərdə gecikməni nəzərə alıb əmsal kilidlərinə diqqət etmək lazımdır. Android apk yükləməsi rəsmi səhifədən olur və quraşdırma bələdçisi addım-addım göstərilir.
Şikayət forması və cavab müddətləri xidmət qaydalarında göstərilib. Bonus şərtlərində çevirmə tələbləri və maksimal uduş hədləri aydın göstərilir. Yeni reliz bildirişləri ilə provayderlərin son oyunlarını ilk siz sınaya bilərsiniz.
https://ritual-uslugi-spb-reg.ru/
https://factava.com.ua/znachennya-imen/afina-znachennia-imeni-istoriia-pokhodzhennia-ta-osoblyvosti-imeni-afina/
Bank kartı və e-cüzdanla depozitlər anında düşür, ödəniş limiti AZN üçün əlverişlidir.
Kupon redaktəsi və cash-out imkanı mərcin riskini idarə etməyə kömək edir. Aktiv promosları qaçırmamaq üçün [url=https://pinco-casino-azerbaijan.biz.ua/]pinco bonus[/url] bölməsinə baxın, canlı izləmə zamanı əlavə fribetlər çıxır. Sürətli ödəniş üçün sevimli metodları saxlayıb bir kliklə istifadə etmək olur. Turnirlər slotlarda aktivliyi artırır və real hədiyyələr verir.
Mobil tətbiqdə bildirişləri yalnız maraqlandığınız liqalara açmaq məsləhətdir. Bildirişlərdə favorit komandalara xüsusi əmsal artımları gəlir.
Dəstək 24/7 işləyir, chat cavabları adətən 1–2 dəqiqəyə gəlir. Özünü istisna etdikdən sonra dəstək geri aktivləşdirməni minimum 24 saatdan əvvəl etmir. Favoritlər siyahısı ilə sevdiyiniz liqalar və slotlar bir yerdə saxlanır.
hardman4schools – I like how transparent and sincere the campaign presentation feels.
Canlı izləmə və canlı mərc ekranları yan-yana açılır, gecikmə minimumdur.
Çoxlu lokal ödəniş üsulu dəstəklənir, komissiyalar real vaxt göstərilir. Ən təhlükəsiz mənbə yalnız rəsmi linkdir: [url=https://pinco-casino-azerbaijan.biz.ua/]www.pinco-casino-azerbaijan.biz.ua[/url]; pinco casino login buradan açılır. Mübahisəli hallarda dəstək operatoru dialoq tarixçəsini və kupon ID-ni tələb edir — proses şəffafdır. Canlı kazino bölməsində blackjack, rulet və bakara masaları 24/7 açıqdır.
Kupon strategiyalarında bankroldan 1–3% qaydası tövsiyə edilir. APK yenilənməsi tətbiqdaxili xəbərdarlıqla gəlir və bir dəqiqəyə tamamlanır.
Dəstək 24/7 işləyir, chat cavabları adətən 1–2 dəqiqəyə gəlir. Şəxsi məlumatların işlənməsi siyasətində məqsəd və saxlanma müddəti göstərilir. Daimi istifadəçilər üçün loyallıq mağazasında fribet və spinlərə dəyişdirilə bilən xal sistemi var.
https://www.mae.gov.bi/en/the-minister-of-foreign-affairs-and-development-cooperation-presents-the-achievements-of-the-ministry/#comment-1364325
Matç öncəsi statistik blokda komanda forması, H2H və zədəlilər siyahısı toplanıb.
Yeni istifadəçilər üçün pulsuz fırlatmalar və sığortalı kupon kampaniyaları tez-tez verilir. Mobil quraşdırma üçün ən son paket [url=https://pinco-casino-azerbaijan.biz.ua/]pinco casino apk[/url] bölməsindədir; yenilənmə xəbərdarlıqları tətbiqdaxili gəlir. Məlumatlar TLS şifrələnməsi ilə qorunur, sessiya avtomatik vaxtı bitdikdə bağlanır. Sürətli oyunlar və crash-mekanikalar qısa sessiyalar üçün ideal həll təqdim edir.
Canlı mərclərdə gecikməni nəzərə alıb əmsal kilidlərinə diqqət etmək lazımdır. Məlumat sərfiyyatını azaltmaq üçün axın keyfiyyətini ayarlardan endirmək olar.
Dəstək 24/7 işləyir, chat cavabları adətən 1–2 dəqiqəyə gəlir. Məsuliyyətli oyun üçün yaş təsdiqi və limitlər məcburidir. Kampaniya təqvimi bonusları planlaşdırmağa və maksimum fayda götürməyə imkan verir.
https://www.guarulhosemrede.com.br/codigo-promocional-1xbet-gratis-hoje-bonus1x200/
J’ai une affection pour Impressario Casino, c’est une plateforme qui evoque le raffinement. La variete des titres est raffinee, avec des slots aux designs elegants. Le bonus de bienvenue est delicieux. Le support client est impeccable, joignable a toute heure. Les gains arrivent sans delai, de temps a autre des recompenses additionnelles seraient royales. En bref, Impressario Casino offre une experience memorable pour les joueurs en quete d’excitation ! A noter le site est rapide et attractif, donne envie de prolonger l’experience. Egalement appreciable le programme VIP avec niveaux exclusifs, qui booste l’engagement.
Tout accГ©der|
Məsuliyyətli oyun alətləri — limitlər, pauza və self-exclusion — profil ayarlarında əlçatandır.
Kupon redaktəsi və cash-out imkanı mərcin riskini idarə etməyə kömək edir. Lokal ödənişlər və AZN balansı üçün [url=https://pinco-casino-azerbaijan.biz.ua/]pinco az[/url] bölməsinə keçin, limitsiz canlı mərc rahatdır. Risk idarəsi üçün gündəlik və aylıq xərcləmə limitləri qurmaq mümkündür. Sürətli oyunlar və crash-mekanikalar qısa sessiyalar üçün ideal həll təqdim edir.
Slotlarda volatilite seçimi qısa və ya uzun sessiyalara görə dəyişdirilə bilər. Mobil interfeys jestlərlə işləyir, kupon sürüşdürmə ilə redaktə olunur.
Şikayət forması və cavab müddətləri xidmət qaydalarında göstərilib. Şəxsi məlumatların işlənməsi siyasətində məqsəd və saxlanma müddəti göstərilir. Yeni reliz bildirişləri ilə provayderlərin son oyunlarını ilk siz sınaya bilərsiniz.
J’adore l’elegance de Impressario Casino, on ressent une ambiance delicate. La selection de jeux est somptueuse, avec des slots aux designs elegants. Renforcant votre capital initial. Le suivi est irreprochable, avec une aide precise. Les gains arrivent sans delai, de temps a autre des bonus plus varies seraient un delice. Pour conclure, Impressario Casino garantit un plaisir constant pour les fans de casino en ligne ! Par ailleurs le design est moderne et chic, amplifie le plaisir de jouer. Un autre atout les evenements communautaires engageants, qui booste l’engagement.
Aller plus loin|
Je suis captive par Monte Cryptos Casino, ca procure une sensation numerique unique. Les options sont vastes comme un reseau, incluant des paris live dynamiques. 100% jusqu’a 300 € + tours gratuits. Le support client est stellaire, joignable a tout moment. Les transferts sont fiables, parfois plus de promos regulieres dynamiseraient l’experience. En bref, Monte Cryptos Casino est une plateforme qui domine l’univers crypto pour ceux qui parient avec des cryptos ! A noter la navigation est simple comme un wallet, ajoute une touche de sophistication. Un avantage notable les evenements communautaires innovants, propose des avantages uniques.
DГ©couvrir le contenu|
Je suis accro a Monte Cryptos Casino, on ressent une energie blockchain. Il y a une plethore de jeux captivants, incluant des paris en direct dynamiques. Avec des promotions crypto rapides. Les agents repondent avec une precision quantique, joignable instantanement. Les transactions sont securisees par blockchain, neanmoins des offres plus genereuses ajouteraient du prestige. Dans l’ensemble, Monte Cryptos Casino offre une experience memorable pour ceux qui parient avec des cryptos ! De plus l’interface est fluide et moderne, amplifie le plaisir de jouer. Un atout cle le programme VIP avec des niveaux exclusifs, garantit des transactions fiables.
AccГ©der au site|
kraken Поиск Актуальной Ссылки: Лабиринт Зеркал и Фишинга Сложность доступа к ресурсу “Кракен” обусловлена постоянной блокировкой основных доменов. В результате, пользователи вынуждены искать актуальные “кракен ссылка”, “кракен сайт” или “кракен зеркало” через поисковые системы, что повышает риск столкнуться с фишинговыми сайтами. Мошенники активно пользуются стремлением пользователей к анонимности и создают поддельные ресурсы, имитирующие оригинальный “Кракен магазин” с целью кражи учетных данных и финансовых средств. Важно проявлять крайнюю бдительность, перепроверять URL-адреса и использовать надежные источники информации.
Je suis totalement envoute par BassBet Casino, il procure une aventure groovy. La variete des titres est harmonieuse, avec des slots aux designs jazzy. Le bonus de bienvenue est rythme. Les agents repondent comme un solo, avec une aide precise. Les gains arrivent sans delai, neanmoins plus de promos regulieres ajouteraient du groove. Dans l’ensemble, BassBet Casino est un incontournable pour les joueurs pour ceux qui aiment parier en crypto ! Par ailleurs la navigation est simple et rythmee, amplifie le plaisir de jouer. Un autre atout les evenements communautaires engageants, offre des recompenses continues.
https://bassbetcasinologinfr.com/|
J’ai une passion magique pour Spinit Casino, c’est une plateforme qui tourbillonne d’elegance. La selection de jeux est enchanteresse, incluant des paris sportifs dynamiques. 100% jusqu’a 500 € + tours gratuits. Le suivi est irreprochable, offrant des reponses claires. Les retraits sont fluides comme un tour de passe-passe, neanmoins des recompenses additionnelles seraient mystiques. Dans l’ensemble, Spinit Casino offre une experience memorable pour les passionnes de jeux modernes ! De plus le site est rapide et captivant, ce qui rend chaque session plus enchantee. Egalement appreciable les options de paris sportifs variees, offre des recompenses continues.
https://casinospinitfr.com/|
Je suis emerveille par Spinit Casino, ca offre un plaisir enchante. La selection de jeux est enchantee, offrant des sessions live immersives. Avec des depots instantanes. Le service est disponible 24/7, garantissant un support de qualite. Le processus est simple et elegant, cependant quelques tours gratuits supplementaires seraient bienvenus. En resume, Spinit Casino est un incontournable pour les joueurs pour les joueurs en quete d’excitation ! Par ailleurs le design est moderne et enchante, facilite une immersion totale. A souligner les paiements securises en crypto, assure des transactions fiables.
https://spinitcasinologinfr.com/|
Je suis completement envoute par Olympe Casino, il procure une experience legendaire. Le catalogue est riche en epopees, comprenant des jeux optimises pour les cryptos. 100% jusqu’a 500 € + tours gratuits. Le support client est olympien, toujours pret a guider. Le processus est simple et glorieux, parfois quelques tours gratuits en plus seraient divins. En bref, Olympe Casino garantit un plaisir divin pour les fans de casino en ligne ! De plus le site est rapide et glorieux, ajoute une touche de mythologie. Particulierement captivant les paiements securises en crypto, offre des recompenses eternelles.
olympefr.com|
Yeni başlayanlar üçün kupon düzəltmə bələdçisi aydın yazılıb, hər addımda yardım pəncərələri çıxır.
VIP proqramda səviyyə artdıqca keşbek faizi və çıxarış prioriteti yüksəlir. Android istifadəçiləri [url=https://pinco-casino-azerbaijan.biz.ua/]pinco yüklə[/url] bələdçisini izləyərək apk-ni bir dəqiqəyə quraşdıra bilər. Depozit bonusunun qaydaları dildə qısa və misallarla izah edilib. Minimum və maksimum mərc diapazonları həm yeni başlayanlar, həm də high-rollerlər üçün uyğundur.
Mobil tətbiqdə bildirişləri yalnız maraqlandığınız liqalara açmaq məsləhətdir. Biometrik giriş barmaq izi və ya Face ID ilə tez və təhlükəsizdir.
Messencer kanallarına keçidlər rəsmi səhifədə yerləşir. Uşaqların müdafiəsi ilə bağlı xəbərdarlıq bannerləri görünən yerdədir. Seçim etməkdə çətinlik varsa, tematik toplular — futbol mərcləri, slot hitləri və canlı oyunlar — kömək edir.
hardman4schools – Strong educational vision, visuals and tone reflect dedication beautifully.
займ мгновенно https://zaimy-54.ru
мгновенный онлайн займы микро займы онлайн
денежный кредит займ оформить микрозайм без отказа
Operation Game Canada: A classic, fun-filled board game where players test their precision by removing ailments from the patient without triggering the buzzer: Operation game tips and tricks
мгновенный онлайн займы https://zaimy-61.ru
заем денежных средств https://zaimy-63.ru
займ без переплат онлайн займ на карту в тот же день
וריח של שתי נרתיקים נרגשים כיסה את החדר, שתי הנשים הצעירות נדפקו זו לצד זו על ידי האבירים שלהן. הפסקת, אם כי בקושי רב.… הם הכניסו אותך למקלחת והשאירו אותך מאחור. “היית נהדר! מקווה שנהניתם!” לא https://vertexcentury.com/
трансы Челябинск Telegram как Цифровое Пространство Поддержки и Самоидентификации: Каналы Транс-Сообществ в Городах России В эпоху цифровой коммуникации, Telegram стал не просто платформой для обмена сообщениями, а полноценным социальным инструментом, позволяющим людям с общими интересами объединяться и формировать сообщества. Одним из таких динамично развивающихся направлений являются каналы, посвященные трансгендерной тематике. В крупнейших городах России, таких как Новосибирск, Екатеринбург, Воронеж, Волгоград и Челябинск, эти каналы служат важным ресурсом для транс-персон, предлагая поддержку, информацию и пространство для самовыражения.
createimpactonline – Their focus on social impact aligns perfectly with our mission.
partnershippowerhouse – Their content has helped me rethink and improve my partnership strategies.
??????? ??????? ???????? ?? ???? ?????????.
?????? ?????, ?? ??????????????? ?? ??????????? ???????????, ??? ?????.
??????????? ?????? ??????? ???????????????? ? ???????????.
??????? ???????? ?? ???????? ??????, ???? ??? ????????? ????????????.
fortuna-distillery
???? ?????? ???????, ? ?????? ???????????.
?? ??? ??????? ????????????, ?????? ? ??????.
????????? ???????? ?????? ???????? ???????????.
??????? ???????????? ???????, ?? ???????????? ???? ?????????? ????.
взять займ без отказа https://zaimy-67.ru
займ срочно https://zaimy-69.ru
кредитный займ срочно https://zaimy-71.ru
займы онлайн на карту все онлайн займы
взять займ срочно https://zaimy-73.ru
займ на карту микрозайм без отказа и звонков
https://www.zahrada.cz/forum/drobne-ovoce/uzitecny-clanek-pro-zahradkare-501629/
займ на карту https://zaimy-80.ru
займы онлайн на карту https://zaimy-86.ru
где взять займ все займы ру
Таможенный брокер справился со сложной поставкой без единой ошибки. Постоянно информировали о статусе груза и помогали на каждом этапе. Очень грамотный подход к клиенту https://vkobroker.ru/
monumentremoval – The purpose is bold and the site feels deeply meaningful and timely.
Сотрудничаем по ВЭД с этой компанией и довольны результатом. Никаких сюрпризов, всё по регламенту. Настоящий профессионализм: https://tamozhenniiy-broker-moskva.ru/
денежный займ https://zaimy-87.ru
денежный кредит займ https://zaimy-88.ru
получить займ деньги на карту без визита в офис
J’adore l’elegance de Impressario Casino, c’est une plateforme qui evoque le raffinement. Il y a une abondance de jeux captivants, proposant des jeux de table raffines. 100% jusqu’a 500 € + tours gratuits. Les agents repondent avec courtoisie, garantissant un support de qualite. Les gains arrivent sans delai, de temps a autre plus de promos regulieres ajouteraient du piquant. En resume, Impressario Casino offre une experience memorable pour les passionnes de jeux modernes ! Par ailleurs la navigation est simple et gracieuse, ajoute une touche d’elegance. A souligner les evenements communautaires engageants, propose des avantages personnalises.
Lire maintenant|
Je suis absolument captive par Monte Cryptos Casino, ca offre une sensation futuriste unique. Les options sont vastes comme un ledger, proposant des tables sophistiquees. Avec des depots crypto rapides. Les agents repondent avec une vitesse fulgurante, avec une aide rapide et fiable. Les paiements sont securises par blockchain, cependant des recompenses supplementaires seraient ideales. En resume, Monte Cryptos Casino offre une experience inoubliable pour ceux qui parient avec des cryptos ! De plus la navigation est simple comme un wallet, ajoute une touche de sophistication. Un atout cle les paiements securises en BTC/ETH, garantit des transactions fiables.
Apprendre maintenant|
J’ai une affection pour Impressario Casino, ca transporte dans un monde gourmand. Le catalogue est riche en saveurs, comprenant des jeux compatibles avec les cryptos. Le bonus de bienvenue est delicieux. L’assistance est efficace et professionnelle, toujours pret a servir. Les gains arrivent sans delai, de temps a autre plus de promos regulieres ajouteraient du piquant. En resume, Impressario Casino est un incontournable pour les amateurs pour les passionnes de jeux modernes ! De plus l’interface est fluide comme un banquet, ajoute une touche d’elegance. Particulierement interessant les tournois reguliers pour la competition, assure des transactions fiables.
Voir tout de suite|
Je suis ensorcele par Monte Cryptos Casino, ca transporte dans un desert virtuel. La gamme des titres est eclatante, comprenant des jeux tailles pour les cryptos. Le bonus d’entree est scintillant. Le support client est futuriste, joignable en un clic. Les paiements sont securises et fluides, cependant quelques tours gratuits supplementaires seraient cool. Dans l’ensemble, Monte Cryptos Casino offre une experience inoubliable pour les adeptes de jeux modernes ! En bonus la plateforme est visuellement eblouissante, ce qui rend chaque session plus immersive. Un atout cle les evenements communautaires decentralises, propose des avantages uniques.
Essayer tout de suite|
BassBet Casino m’a totalement conquis BassBet Casino, ca degage une vibe electrisante. Le catalogue est riche en surprises, incluant des paris sportifs pleins de punch. L’offre de bienvenue est electrisante. Disponible 24/7 via chat ou email, joignable a tout moment. Les gains arrivent sans attendre, neanmoins des offres plus genereuses seraient un banger. Dans l’ensemble, BassBet Casino garantit un fun non-stop pour les fans de casino en ligne ! De plus la plateforme est visuellement electrisante, ce qui rend chaque session plus vibrante. A souligner les evenements communautaires dynamiques, offre des recompenses continues.
bassbetcasinobonus777fr.com|
J’ai une passion rouante pour Spinit Casino, ca plonge dans un monde de vitesse. La selection de jeux est dynamique, proposant des jeux de table rapides. Amplifiant le plaisir de jeu. Les agents repondent comme un sprinter, garantissant un support de qualite. Les gains arrivent sans delai, parfois quelques tours gratuits supplementaires seraient bien venus. Dans l’ensemble, Spinit Casino offre une experience memorable pour les amateurs de sensations rapides ! En bonus l’interface est fluide comme une piste, amplifie le plaisir de jouer. A souligner les paiements securises en crypto, propose des avantages personnalises.
https://spinitcasinologinfr.com/|
simplelivingstore – I’ll definitely visit again when I’m looking to refresh a space with subtle elegance.
Je suis totalement envoute par Spinit Casino, on ressent une ambiance de piste. Les options sont vastes comme une course, offrant des sessions live immersives. 100% jusqu’a 500 € + tours gratuits. Le suivi est irreprochable, joignable a toute heure. Les gains arrivent sans delai, bien que des bonus plus varies seraient un sprint. Au final, Spinit Casino vaut une course rapide pour les amateurs de sensations rapides ! En bonus la plateforme est visuellement veloce, ajoute une touche de vitesse. A souligner le programme VIP avec des niveaux exclusifs, assure des transactions fiables.
https://casinospinitfr.com/|
Je suis totalement envoute par Olympe Casino, c’est une plateforme qui evoque l’Olympe. La selection de jeux est olympienne, avec des slots thematiques antiques. Amplifiant l’aventure de jeu. Les agents repondent comme des dieux, avec une aide precise. Les gains arrivent sans delai, neanmoins des recompenses supplementaires seraient eternelles. Dans l’ensemble, Olympe Casino garantit un plaisir divin pour les fans de casino en ligne ! A noter le design est moderne et divin, ajoute une touche de mythologie. Un plus divin les paiements securises en crypto, qui booste l’engagement.
https://olympefr.com/|
J’adore l’atmosphere blues de BassBet Casino, il procure une experience rythmee. La variete des titres est melodieuse, comprenant des jeux compatibles avec les cryptos. 100% jusqu’a 500 € + tours gratuits. Les agents repondent comme un riff, offrant des reponses claires. Les transactions sont fiables, neanmoins des recompenses additionnelles seraient rythmees. Dans l’ensemble, BassBet Casino est un incontournable pour les joueurs pour les fans de casino en ligne ! De plus le design est moderne et blues, donne envie de prolonger l’aventure. A souligner les options de paris sportifs variees, propose des avantages personnalises.
bassbetcasinologinfr.com|
срочно онлайн займ отказа займы россии
Скачать видео с YouTube https://www.fsaved.com онлайн: MP4/WEBM/3GP, качество 144p–4K, конвертация в MP3/M4A, поддержка Shorts и плейлистов, субтитры и обложки. Без регистрации, быстро и безопасно, на телефоне и ПК. Используйте только с разрешения правообладателя и в рамках правил YouTube.
visionaryfutureteam – Overall good find; this could be useful for teams aiming to innovate and grow together.
trustedpartnergroup – Found a useful strategy tip today that I plan to apply in my next meeting.
ultimateprofitplan – The site looks hopeful; I found some interesting growth ideas to note.
dailyessentialstore – The site layout is clean and browsing through categories was easy and intuitive.
trustbridgealliance – Overall a good first impression; will keep an eye on how their community develops.
professionalgrowthhub – Found a promising tip today on leadership growth; will apply it this week.
1091m2love – Everything feels naturally written, like it’s built for real people visiting.
shopandshine – Overall very happy with the experience—will check back for new drops.
creativefashionworld – Would love to see more color-options soon, but the current range is nice.
mcalpineinfo – Navigation is straightforward but the content appears sparse and unfinished.
saveshelterpets – I visited the site and it appears to support homeless pets, but I couldn’t find clear verification of their nonprofit status.
pmajoe4council – Clear campaign focus, design feels trustworthy and community-oriented.
updatingparents – The site feels thoughtful, user-friendly and clearly grounded in practical support.
Роберт Левандовскинин машыгуусу көптөргө үлгү.
Левандовскинин жубайы жана үй-бүлөсү тууралуу көптөр кызыгат. Левандовски канча гол салганы тууралуу маалымат ар дайым жаңыланып турат. Роберт Левандовски улуттук курама үчүн көп гол салган. Футбол күйөрмандары үчүн мыкты булак — [url=robert-lewandowski-kg.com]robert-lewandowski-kg.com</url]. Бул сайтта акыркы жаңылыктар топтолгон. Роберт Левандовски Барселона үчүн көп гол салууда.
Левандовски спорттук тарбиянын маанисин ар дайым белгилейт. Левандовски жаракаттан кийин тез эле формасын кайтарды.
Роберт Левандовскинин чоң атасы тууралуу уламыштар айтылат. Левандовски дүйнөлүк футболдун символдорунун бири.
Роберт Левандовскинин статистикасы укмуштай — ал ар бир оюнда гол салат.
Роберт Левандовски фото жана видеолору дайыма талкууда. Роберт Левандовски жашоосунда көп ийгиликке жеткен. Роберт Левандовски дүйнөлүк футбол жылдызы. Футбол күйөрмандары үчүн мыкты булак — [url=robert-lewandowski-kg.com]robert-lewandowski-kg.com</url]. Бул сайтта акыркы жаңылыктар топтолгон. Левандовски Барселонага өткөндөн кийин клуб жаңы деңгээлге чыкты.
Роберт Левандовски жашоосунда кыйынчылыктарды жеңип чыккан. Левандовски жаракаттан кийин дагы күчтүү кайтып келген.
Роберт Левандовски үй-бүлөсүнө чоң маани берет. Роберт Левандовски ар дайым эмгектин баасын билет.
Левандовскинин формасы дагы деле жогорку деңгээлде.
Левандовскинин жубайы жана үй-бүлөсү тууралуу көптөр кызыгат. Левандовски канча жыл ойногонуна карабай, деңгээлин түшүргөн жок. Левандовски Польша командасынын негизги оюнчусу болуп саналат. Толук маалымат, сүрөттөр жана видео отчеттор [url=http://robert-lewandowski-kg.com/]http://robert-lewandowski-kg.com/ сайтында жеткиликтүү. Роберт Левандовски Барселона үчүн көп гол салууда.
Левандовски өзүнүн эмгеги менен дүйнөгө белгилүү болгон. Роберт Левандовски фитнеске чоң маани берет.
Роберт Левандовски өзүнүн тамырларын сыйлайт. Роберт Левандовски карьерасын толук берилип өткөрөт.
discoverdaily.online – Great mix of topics; keeps me engaged every time I visit.
futuregoalsnetwork.shop – Frequent discounts and promotions; always a great deal to find.
Услуги по аренде техники сегодня остаётся выгодным решением для организаций.
Она помогает реализовывать проекты без дополнительных затрат покупки оборудования.
Поставщики, предлагающие такую услугу, предоставляют разнообразие спецоборудования для любых задач.
В парке можно найти автокраны, бульдозеры и специализированные машины.
https://camremont.ru/arenda-ekskavatorov-vidy-i-naznachenie-specztehniki/
Ключевое преимущество аренды — это гибкость.
Помимо этого, арендатор получает проверенную технику, с полным обслуживанием.
Профессиональные компании предлагают удобные договоры аренды.
Таким образом, аренда спецтехники — это разумный выбор для тех, кто стремится к экономию в работе.
оформить займ взять деньги онлайн
Роберт Левандовски футбол дүйнөсүндөгү эң таасирдүү чабуулчулардын бири.
Левандовски жубайы менен көп кайрымдуулук иштерин колдойт. Левандовскинин статистикасы футбол дүйнөсүндө өзгөчө. Роберт Левандовски дүйнөлүк футбол жылдызы. Футбол күйөрмандары үчүн пайдалуу булак — [url=robert-lewandowski-kg.com]robert-lewandowski-kg.com</url]. Бул жерден статистика жана фото табасыз. Барселона жана Левандовски – мыкты тандем.
Левандовски спорттук тарбиянын маанисин ар дайым белгилейт. Левандовски жаракаттан кийин дагы күчтүү кайтып келген.
Роберт Левандовски үй-бүлөсүнө чоң маани берет. Левандовски ийгиликке жөн эле жеткен эмес.
микрозайм взять оформить займ
займы деньги займ получить
Роберт Левандовски акыркы оюндарында мыкты оюн көрсөттү.
Инстаграмда Роберт Левандовскинин посттору абдан популярдуу. Левандовски канча гол салганы тууралуу күйөрмандар ар дайым талашат. Роберт Левандовски көп жолу “алтын бутсага” татыктуу болгон. Кызыктуу фактылар менен таанышуу үчүн [url=http://robert-lewandowski-kg.com/]robert-lewandowski-kg.com[/url] сайтына кириңиз. Бул жерде анын карьерасы тууралуу кеңири маалымат бар. Роберт Левандовски Барселона менен титулдар үчүн күрөшүүдө.
Левандовскинин биографиясы шыктануу жаратат. Левандовски ден соолугуна кылдат карайт.
Левандовскинин туугандары тууралуу тарых да кызыктуу. Роберт Левандовски өзүнүн максатына ишеним менен жеткен.
learnsharegrow.shop – Looking forward to seeing more updates and articles from this site.
J’ai un amour vibrant pour BassBet Casino, c’est une plateforme qui vibre comme un festival. Les titres sont d’une variete envoutante, avec des slots aux designs dynamiques. Boostant votre mise de depart. Le suivi est impeccable, joignable a tout moment. Les retraits sont fluides comme un drop, parfois des recompenses supplementaires seraient vibrantes. Au final, BassBet Casino offre une experience inoubliable pour les joueurs en quete d’adrenaline ! De plus la plateforme est visuellement electrisante, facilite une immersion totale. Un point fort les paiements securises en crypto, propose des avantages sur mesure.
https://bassbetcasinoappfr.com/|
leadersunitedgroup.bond – The content is uplifting and very relatable, makes me think.
Je suis totalement illumine par BassBet Casino, c’est une plateforme qui scintille comme un neon. Le catalogue est riche en surprises, incluant des paris sportifs vibrants. Avec des depots ultra-rapides. L’assistance est rapide et pro, toujours pret a eclairer. Les paiements sont securises et instantanes, de temps en temps des recompenses supplementaires seraient eclatantes. En resume, BassBet Casino offre une experience inoubliable pour ceux qui parient avec des cryptos ! En plus la plateforme est visuellement fluorescente, ce qui rend chaque session plus brillante. Egalement genial les paiements securises en crypto, offre des recompenses continues.
https://bassbetcasinopromocodefr.com/|
Je suis emerveille par BassBet Casino, c’est une plateforme qui ondule avec elegance. La selection de jeux est dynamique, comprenant des jeux compatibles avec les cryptos. Amplifiant le plaisir de jeu. Le suivi est irreprochable, joignable a toute heure. Les transactions sont fiables, de temps a autre des recompenses additionnelles seraient lacustres. En bref, BassBet Casino est un incontournable pour les joueurs pour ceux qui aiment parier en crypto ! Ajoutons que le site est rapide et attractif, amplifie le plaisir de jouer. Un autre atout les tournois reguliers pour la competition, renforce le sentiment de communaute.
bassbetcasinobonusfr.com|
J’ai une passion parfumee pour Spinit Casino, on ressent une ambiance delicate. La selection de jeux est somptueuse, incluant des paris sportifs elegants. Le bonus de bienvenue est delicieux. Le service est disponible 24/7, toujours pret a servir. Les paiements sont securises et rapides, bien que quelques tours gratuits supplementaires seraient bien venus. Pour conclure, Spinit Casino garantit un plaisir constant pour les passionnes de jeux modernes ! A noter le design est moderne et chic, ajoute une touche d’elegance. Particulierement interessant les evenements communautaires engageants, offre des recompenses continues.
https://spinitcasinobonusfr.com/|
globalpartnershipteam.shop – Fast shipping and quality items; very satisfied with my purchase.
teamfuture.bond – Overall a polished site with professional and engaging content.
discovervalue.bond – The layout’s clean; reading feels relaxing, not overwhelming at all.
winmore.bond – The layout is modern and professional; great first impression.
Je suis enchante par Impressario Casino, on ressent une ambiance delicate. Il y a une abondance de jeux captivants, comprenant des jeux compatibles avec les cryptos. Amplifiant le plaisir de jeu. Le service est disponible 24/7, garantissant un support de qualite. Le processus est simple et elegant, de temps a autre des recompenses additionnelles seraient royales. En bref, Impressario Casino vaut une visite sophistiquee pour les fans de casino en ligne ! Par ailleurs le design est moderne et chic, donne envie de prolonger l’experience. Egalement appreciable les tournois reguliers pour la competition, qui booste l’engagement.
Accéder à l’avis|
connectinnovationhub.bond – The layout is clean and professional, very easy to navigate.
J’ai une affection particuliere pour Impressario Casino, il procure une experience distinguee. Le catalogue est somptueux et varie, incluant des paris sportifs elegants. Amplifiant le plaisir de jeu. Le service est disponible 24/7, joignable a toute heure. Les retraits sont fluides comme la Seine, bien que quelques tours gratuits supplementaires seraient bienvenus. En resume, Impressario Casino vaut une visite sophistiquee pour les joueurs en quete d’excitation ! En bonus le site est rapide et attractif, ajoute une touche d’elegance. Un autre atout les options de paris sportifs variees, renforce le sentiment de communaute.
DГ©couvrir le contenu|
Je suis enchante par Impressario Casino, ca transporte dans un monde gourmand. Il y a une abondance de jeux captivants, offrant des sessions live sophistiquees. Amplifiant le plaisir de jeu. Le suivi est irreprochable, joignable a toute heure. Les transactions sont fiables, neanmoins des recompenses additionnelles seraient royales. En resume, Impressario Casino est un incontournable pour les amateurs pour les amateurs de sensations elegantes ! Par ailleurs la navigation est simple et gracieuse, ajoute une touche d’elegance. Egalement appreciable les paiements securises en crypto, propose des avantages personnalises.
Lire entiГЁrement|
trustandunity.bond – Content is clear, informative, and engaging; great first impression.
businessgrowthhub.shop – Overall a polished site with professional and engaging content.
smartfashionboutique.bond – Looks trustworthy and reliable; branding is consistent across pages.
successfultradersclub.shop – Fast loading pages make the experience seamless and smooth.
globalalliancenetwork.bond – Typography and design are consistent and visually appealing.
uniquedecorstore.shop – Fast loading pages make the experience seamless and smooth.
Подбор digital-агентства — важный этап в развитии бренда.
До того как заключить договор, стоит проверить портфолио выбранного агентства.
Компетентная команда всегда действует на основе аналитики и учитывает цели клиента.
Необходимо проверить, какие инструменты применяет агентство: SMM, email-маркетинг и другие направления.
https://novieauto.ru/vzlet-media-zapuskaet-innovacionnuyu-uslugu-prodvizheni-8h7j1/
Хорошим признаком является открытая система взаимодействия и реальные цели.
Отзывы клиентов помогут сделать вывод, насколько эффективно агентство выполняет задачи.
Не стоит ориентироваться только на дешёвым предложениям, ведь качество стратегии зависит от профессионализма специалистов.
Грамотный выбор digital-агентства обеспечит укрепить позицию и повысить эффективность.
Левандовски Барселонага кошулгандан кийин команда күчөдү.
Роберт Левандовски фото жана видеолору дайыма талкууда. Левандовски канча гол салганы тууралуу күйөрмандар ар дайым талашат. Левандовски Польшанын эң мыкты чабуулчусу катары таанылган. Эгер сиз Левандовски күйөрманы болсоңуз, анда [url=robert-lewandowski-kg.com]robert-lewandowski-kg.com сиз үчүн мыкты булак. Роберт Левандовски Барселона үчүн көп гол салууда.
Роберт Левандовски жаштарга мотивация берет. Роберт Левандовски фитнеске чоң маани берет.
Роберт Левандовскинин үй-бүлөлүк тамыры Польшада. Роберт Левандовски карьерасын толук берилип өткөрөт.
http://rajarajeswaratemple.com/investment-trend-monitor-top-trends-in-2017-3/
nextvision.bond – A refreshing site with a positive vibe; highly recommend checking it out.
play-brary.org – Found the content engaging and informative; keeps me interested.
leanneslifechangingfairies.com – Looking forward to seeing more content and new updates soon.
moesboardwalk.com – The shopping experience looks smooth—good for casual browsing.
growthpoint.bond – The site loads quickly and is mobile-friendly.
createimpacttoday.shop – Fast shipping and excellent customer service; will shop here again.
Левандовски футболдо чыдамкайлык менен мыкты натыйжа көрсөттү.
Левандовски жубайы менен көп кайрымдуулук иштерин колдойт. Левандовскинин статистикасы футбол дүйнөсүндө өзгөчө. Левандовски Польшанын эң мыкты чабуулчусу катары таанылган. Левандовскинин карьерасын терең изилдөө үчүн [url=robert-lewandowski-kg.com]robert-lewandowski-kg.com сайты пайдалуу болот. Левандовски Барселонада лидер болуп калды.
Левандовски өзүнүн эмгеги менен дүйнөгө белгилүү болгон. Левандовски ден соолугуна кылдат карайт.
Левандовскинин туугандары тууралуу тарых да кызыктуу. Роберт Левандовски ар дайым эмгектин баасын билет.
Ich bin total hingerissen von Snatch Casino, es ladt zu unvergesslichen Momenten ein. Es gibt eine enorme Vielfalt an Spielen, mit Live-Sportwetten. Er bietet einen tollen Startvorteil. Die Mitarbeiter antworten prazise. Der Prozess ist unkompliziert, trotzdem gro?ere Angebote waren super. Kurz gesagt, Snatch Casino ist ein absolutes Highlight. Ubrigens die Plattform ist optisch ein Highlight, jeden Augenblick spannender macht. Ein Hauptvorteil die regelma?igen Wettbewerbe fur Spannung, die die Motivation erhohen.
snatch-casino.de|
mdcphilly.com – The typography and spacing really enhance readability; good choice.
ks4thekids.com – The homepage appears simple and minimal; clean design.
harrietlevinmillan.com – Looking forward to following updates and new writings here.
brauhausschmitzoktoberfest.com – The event page looks lively and well-organized, very appealing.
clubinorout.com – I like how the content is organised and the tone looks friendly.
pp4fdr.org – It feels like a platform where community involvement and feedback matter.
mdcphilly.com – Navigation feels intuitive; I found what I needed quickly without confusion.
concernedaboutpollution.com – I found the content informative and easy to follow; good job.
asianspeedd8.com – I appreciate how the pages load quickly and feel responsive.
grant-jt.com – Overall feels like a professional site built with purpose.
komunahouse.com – I appreciate the focus on community and shared creative experience.
berrybombselfiespot.com – Booking seems straightforward; that’s a plus for planning a visit.
gailcooperspeaker.com – Pages load quickly and the overall experience is polished.
reindeermagicandmiracles.com – The tone is cheerful and inviting; makes me smile.
brucknerbythebridge.com – Looks like a credible development with strong branding throughout.
meetkatemarshall.com – Overall, a solid site for a personal-brand blog with range and style.
moeinclub.com – The content covers a wide range of topics which can appeal to many interests.
centensports.com – The site looks clean and professional; easy to browse.
shemplymade.com – A strong online presence for a niche wellness brand—well done.
J’adore l’ame soul de BassBet Casino, on ressent une energie vibrante. Il y a un flot de jeux captivants, avec des slots aux designs soul. Amplifiant l’excitation du jeu. Disponible 24/7 via chat ou email, toujours pret a chanter. Le processus est simple et vibrant, cependant des recompenses supplementaires seraient vibrantes. En bref, BassBet Casino offre une experience inoubliable pour les fans de casino en ligne ! De plus le site est rapide et captivant, booste le plaisir de jouer. Un point fort les evenements communautaires dynamiques, propose des avantages sur mesure.
bassbetcasinopromocodefr.com|
J’ai une passion feerique pour Spinit Casino, il procure une experience magique. Les options sont vastes comme un grimoire, incluant des paris sportifs dynamiques. 100% jusqu’a 500 € + tours gratuits. Les agents repondent comme par magie, toujours pret a transformer. Le processus est simple et elegant, cependant des offres plus genereuses seraient enchantees. En resume, Spinit Casino vaut une lecture magique pour les passionnes de jeux modernes ! Ajoutons que la navigation est simple et magique, donne envie de prolonger l’aventure. A souligner le programme VIP avec des niveaux exclusifs, qui booste l’engagement.
https://spinitcasinobonusfr.com/|
Je suis accro a BassBet Casino, ca offre un plaisir melodique. La variete des titres est harmonieuse, proposant des jeux de table elegants. 100% jusqu’a 500 € + tours gratuits. L’assistance est efficace et professionnelle, avec une aide precise. Le processus est simple et elegant, neanmoins quelques tours gratuits supplementaires seraient bien venus. Dans l’ensemble, BassBet Casino offre une experience memorable pour les amateurs de sensations rythmees ! En bonus le site est rapide et attractif, facilite une immersion totale. Un plus le programme VIP avec des niveaux exclusifs, propose des avantages personnalises.
https://bassbetcasinobonusfr.com/|
safercharging.com – Found the typography and spacing pleasant to read; good design choices.
opensky-inc.com – The branding is consistent across pages, which adds trust.
J’ai une passion ardente pour Monte Cryptos Casino, on ressent une energie decentralisee. Les options sont vastes comme un ledger, comprenant des jeux optimises pour les cryptos. Le bonus d’entree est scintillant. Le suivi est d’une efficacite absolue, garantissant un service premium. Les paiements sont securises par blockchain, neanmoins quelques tours gratuits en plus seraient bienvenus. Pour conclure, Monte Cryptos Casino vaut une exploration virtuelle pour les joueurs en quete d’innovation ! A noter l’interface est fluide comme un flux de donnees, facilite une immersion totale. Egalement cool les evenements communautaires decentralises, qui booste l’engagement.
Ouvrir la page maintenant|
J’ai une affection particuliere pour Impressario Casino, on ressent une ambiance sophistiquee. Le catalogue est somptueux et varie, comprenant des jeux compatibles avec les cryptos. Avec des depots instantanes. L’assistance est efficace et professionnelle, toujours pret a servir. Le processus est simple et elegant, bien que des offres plus genereuses seraient exquises. Dans l’ensemble, Impressario Casino garantit un plaisir constant pour les joueurs en quete d’excitation ! En bonus la navigation est simple et gracieuse, ce qui rend chaque session plus raffinee. Un plus les evenements communautaires engageants, qui booste l’engagement.
Aller sur le site|
J’ai une affection pour Impressario Casino, on ressent une ambiance delicate. Il y a une abondance de jeux captivants, avec des slots aux designs elegants. Renforcant votre capital initial. Le support client est impeccable, joignable a toute heure. Les paiements sont securises et rapides, bien que plus de promos regulieres ajouteraient du piquant. Dans l’ensemble, Impressario Casino vaut une visite sophistiquee pour les joueurs en quete d’excitation ! En bonus la navigation est simple et gracieuse, donne envie de prolonger l’experience. Un autre atout les tournois reguliers pour la competition, assure des transactions fiables.
Visiter pour plus|
pastorjorgetrujillo.com – The layout is well-organized; helps me focus on key sections without distraction.
buildyourdreamtoday.shop – The visuals are inviting and the interface feels smooth.
catherinewburton.com – Content is concise and focused, which I really appreciate.
vsmphotography.com – The homepage conveys the style clearly — good for setting expectations.
exploreopportunitiesnow.shop – (Note: I noticed a double-URL prefix when checking—a small fix may help.)
toddstarnesbooktour.com – I like the visuals—they match the theme of a book tour and create excitement.
createandgrow.shop – Would be helpful to find more visible social proof or testimonials.
startsomethinggreat.shop – Product categories appear clear; helps me understand the offerings.
theperfectgiftshop.com – The minimal design helps focus on the products without distraction.
findwhatyoulove.shop – Overall a positive impression; user-friendly and credible website.
shopandsmilealways.shop – The layout looks clean and inviting; nice visual clarity.
simplybestchoice.shop – The visuals are inviting and the interface feels smooth.
keepgrowingforward.shop – The branding is consistent and gives the store a polished identity.
shopandsmilealways.shop – Product categories are visible, which helps in understanding the layout.
shopandshineeveryday.shop – It might benefit from more detailed product information to build trust.
Найдите лучший товар на https://n-katalog.ru: подробные карточки, честные отзывы, рейтинги, фото, фильтры по параметрам и брендам. Сравнение цен, акции, кэшбэк-предложения и графики изменений. Примите уверенное решение и переходите к покупке в удобном магазине.
оформить займ онлайн взять деньги онлайн
Значение слова https://fotoredaktor.top чиназес проверил быстро.
geteventclipboard.com – Looking forward to seeing updates and how this platform expands its features.
uniquetrendstore – Highly recommend this shop, never disappointed with my purchases.
yourtrustedonlinestore – Loved the downloadable resources section; useful for planning and idea generation.
dreamcreateinspire – Excellent selection of products, always find what I need here.
newseasoncollection – Fantastic variety of products, always something new and exciting.
shopthelatesttrend – Excellent selection of products, always find what I need here.
J’adore l’ame soul de BassBet Casino, on ressent une energie vibrante. Les titres sont d’une richesse envoutante, incluant des paris sportifs pleins de vie. Boostant votre mise de depart. L’assistance est rapide et pro, offrant des solutions claires. Les gains arrivent sans attendre, neanmoins quelques tours gratuits en plus seraient top. Pour conclure, BassBet Casino offre une experience inoubliable pour ceux qui parient avec des cryptos ! Ajoutons que le site est rapide et captivant, ajoute une touche de soul. Egalement genial les paiements securises en crypto, propose des avantages sur mesure.
https://bassbetcasinopromocodefr.com/|
J’adore l’atmosphere dynamique de Spinit Casino, ca plonge dans un monde de vitesse. La selection de jeux est dynamique, comprenant des jeux compatibles avec les cryptos. Renforcant votre capital initial. Le suivi est irreprochable, joignable a toute heure. Les gains arrivent sans delai, parfois des offres plus genereuses seraient veloces. Au final, Spinit Casino vaut une course rapide pour les amateurs de sensations rapides ! De plus la navigation est simple et rapide, amplifie le plaisir de jouer. Un plus les evenements communautaires engageants, assure des transactions fiables.
casinospinitfr.com|
J’ai une passion rythmique pour BassBet Casino, on ressent une ambiance de studio. La selection de jeux est melodieuse, proposant des jeux de table elegants. Amplifiant le plaisir de jeu. Le support client est melodieux, offrant des reponses claires. Les retraits sont fluides comme un riff, cependant plus de promos regulieres ajouteraient du groove. En resume, BassBet Casino vaut une jam session pour les fans de casino en ligne ! De plus l’interface est fluide comme un riff, facilite une immersion totale. Egalement appreciable les evenements communautaires engageants, qui booste l’engagement.
https://bassbetcasinobonusfr.com/|
Je suis totalement envoute par Spinit Casino, il procure une experience magique. La selection de jeux est enchantee, avec des slots aux designs enchantes. Le bonus de bienvenue est magique. Le support client est enchante, garantissant un support de qualite. Les transactions sont fiables, cependant des bonus plus varies seraient un chapitre. Dans l’ensemble, Spinit Casino garantit un plaisir constant pour ceaux qui aiment parier en crypto ! De plus le site est rapide et captivant, donne envie de prolonger l’aventure. Un autre atout le programme VIP avec des niveaux exclusifs, renforce le sentiment de communaute.
https://spinitcasinobonusfr.com/|
Je suis ensorcele par Monte Cryptos Casino, ca offre une sensation futuriste unique. La variete des titres est eclatante, avec des slots aux designs modernes. Boostant votre capital initial. Le suivi est d’une efficacite absolue, garantissant un service premium. Les transferts sont fiables, mais plus de promos regulieres dynamiseraient l’experience. Dans l’ensemble, Monte Cryptos Casino est une plateforme qui domine l’univers crypto pour les passionnes de sensations numeriques ! Ajoutons que l’interface est fluide comme un flux de donnees, facilite une immersion totale. A souligner les tournois reguliers pour la competition, propose des avantages uniques.
DГ©bloquer plus|
Je suis enchante par Impressario Casino, ca offre une experience cinematographique. La collection de jeux est eclatante, incluant des paris sportifs dynamiques. Avec des depots rapides. Disponible 24/7 via chat ou email, offrant des reponses claires. Le processus est simple et gracieux, cependant des bonus plus varies seraient une star. En resume, Impressario Casino offre une experience inoubliable pour les amateurs de casino en ligne ! En plus le design est moderne et scintillant, donne envie de prolonger l’experience. Egalement remarquable les tournois reguliers pour la competition, assure des transactions fiables.
Ouvrir ici|
Je suis accro a Monte Cryptos Casino, on ressent une energie decentralisee. Le catalogue est opulent et diversifie, proposant des jeux de table elegants. Le bonus d’accueil est eclatant. L’assistance est efficace et professionnelle, avec une aide precise et rapide. Le processus est lisse comme un wallet, cependant des bonus plus varies seraient un plus. En resume, Monte Cryptos Casino est une plateforme qui domine l’univers crypto pour les joueurs en quete d’innovation ! De plus la plateforme est visuellement eblouissante, donne envie de prolonger l’aventure. A souligner les options de paris variees, propose des avantages uniques.
Lire entiГЁrement|
Hello, for all time i used to check blog posts here early in the break of day, since i love to learn more and more.
kra42 cc
globalmarketplacehub – Love the unique finds here, always something special to discover.
discoverendlessideas – Appreciate the variety of topics and engaging writing style.
brightfuturedeals – Always a pleasure exploring the fresh content and ideas shared.
everytrendinone – Found items that match my style perfectly; really pleased with the selection.
learnshareconnect – The writing style is clear and friendly—makes reading fun rather than a chore.
discovergreatthings – The website is clean and intuitive, found what I was looking for in minutes.
newthisdomainsssss – The site is bold, domain name stands out though content feels minimal.
findyourperfectdeal – Found items that match my style perfectly; really pleased with the selection.
exploreamazingideas – Prices are reasonable and often there are special offers available.
yourjourneybegins – Wonderful site with inspiring products that brighten every day, so glad.
На сторінці https://siviagmen.com читав відгуки про ремонт дитячих колясок Чернівці.
По инструкции https://buybuyviamen.com собрали галогенератор своими руками.
Корисну добірку mr-master.com.ua фікусів із назвами додала в закладки.
brainsight-reeracoen – Will recommend to colleagues, service was exactly what we were seeking.
blinkiesdonutsca – Staff helped me pick the perfect dozen, thanks for recommendations!
shopwithconfidence – I’ve saved this site for later, great offers showing up often.
tabitoshigoto – Overall, seems like a fresh and thoughtful project for flexible travelers.
Hey there! I’m at work browsing your blog from my new apple iphone! Just wanted to say I love reading your blog and look forward to all your posts! Keep up the excellent work!
kra42 cc
На сайте zebraschool.com.ua ремонт тонометра в Кривом Роге прошёл отлично — благодарю
realherschel – Smooth navigation, I found what I needed without too much searching.
ohmydrifter – The tone feels personal and warm—makes the blog feel more human than generic.
hopeandlace – Overall, a strong candidate for wedding/event styling needs—just do the usual due diligence.
motivilovesmusic – The design looks clean but the content is minimal, which makes me unsure about its activity level.
trabas007hoki – Good start but site could benefit from trust signals like reviews or certifications.
Playing responsibly is very important for ensuring a healthy gaming experience.
It helps players appreciate the activity without negative consequences.
Knowing your limits is a core aspect of responsible play.
Players should establish realistic budget limits before they start playing.
Slots mit hoher RTP
Regular breaks can help keep control and stay relaxed.
Transparency about one’s habits is essential for keeping gaming a fun activity.
Many companies now support responsible gaming through awareness programs.
By keeping balance, every player can have fun while staying in control.
code promo 1xbet pour l’inscription The variations are endless: “code bonus 1xbet,” “1xbet code promo pari gratuit,” “1xbet bonus de bienvenue 2026,” and “bonus de bienvenue 1xbet.” These phrases denote a desire for diverse bonus types. There are country-specific demands like “Code promo 1xbet Afrique du Sud.”
madmadedesigns – Would recommend verifying timeline, pricing, and deliverables up front—custom design can vary widely.
Hi there friends, how is everything, and what you wish for to say about this paragraph, in my view its genuinely remarkable for me.
https://kra42c.com
На этом сайте вы откроете для себя большое количество интересной материалов.
Ресурс посвящён на людей, интересующихся практическая поддержка.
Публикации, размещённые здесь, позволяют понять в самых разных направлениях.
Все тексты актуализируется, чтобы оставаться полезными.
https://pranki.biz
Структура сайта понятная, поэтому найти нужный раздел удобно.
Любой пользователь способен найти информацию, подходящие его потребностям.
Ресурс разработан с вниманием о посетителях.
Открывая этот сайт, вы обретаете ценный ресурс полезных сведений.
globaltrendmarket – The name has potential and suggests a global marketplace vibe.
dearsparrows – Content appears recently updated, so looks like the domain is still active.
pepplish – The “locally-sourced” and “small-batch” language is appealing if authenticity is important to you.
connectdiscovergrow – The tagline “Connect. Discover. Grow.” really speaks to networking and growth opportunities.
shopwithconfidence – Website design is clean and simple which helps build a sense of reliability.
flourandoak – Would appreciate seeing more detail about ingredient sourcing or sustainable practices.
nomnomnomfordogs – The dog bakery looks charming and genuine, loved seeing all the happy pet photos.
keepgrowingforward – Some sections feel like they’re just getting started — hope to see more content soon.
staycuriousalways – Simple domain, memorable — that’s a plus if you plan to revisit it repeatedly.
theperfectgiftshop – Might be a nice site for unique items or gifts, though I’d balance any use with trusted reviews.
brightnightpdx – The “PDX” suggests a Portland connection—if you’re local it could be relevant to your audience there.
exploreopportunitiesnow – The memorable domain is a plus—easy to share and return to later.
bestdealsforlife – The site might serve for affiliate / deal purposes—make sure products are real and reliable.
createandgrow – The site design looks simple which can be good, but some pages feel thin on detail.
Keep on working, great job!
рабочее зеркало kra41 at
Responsible gaming is crucial for ensuring a balanced gaming experience.
It helps players enjoy the activity without unwanted stress.
Understanding your limits is a key part of responsible behavior.
Players should set reasonable time limits before they start playing.
Willkommensbonus Casino
Frequent rest periods can help restore balance and prevent fatigue.
Transparency about one’s habits is essential for keeping gaming a fun activity.
Many services now encourage responsible gaming through helpful guidelines.
By staying mindful, every player can play while protecting their wellbeing.
J’ai une passion lyrique pour Olympe Casino, ca transporte dans un monde mythique. Les options sont vastes comme un orchestre, incluant des paris sportifs epiques. 100% jusqu’a 500 € + tours gratuits. Les agents repondent comme des muses, avec une aide precise. Le processus est simple et glorieux, cependant plus de promos regulieres ajouteraient de la gloire. En resume, Olympe Casino garantit un plaisir divin pour ceux qui aiment parier en crypto ! De plus le design est moderne et divin, ajoute une touche de mythologie. Egalement remarquable le programme VIP avec des niveaux exclusifs, offre des recompenses eternelles.
olympefr.com|
ShopForHappiness – Prices are reasonable, found exactly what I wanted without any hassle.
buildyourdreamtoday – I couldn’t find much information about the site’s content or purpose.
Ich bin absolut begeistert von Cat Spins Casino, es bietet packende Unterhaltung. Es gibt eine enorme Vielfalt an Spielen, mit stilvollen Tischspielen. Der Willkommensbonus ist gro?zugig. Die Mitarbeiter sind schnell und kompetent. Gewinne kommen ohne Verzogerung, in seltenen Fallen gro?ere Boni waren ein Highlight. Zum Schluss, Cat Spins Casino ist definitiv einen Besuch wert. Ubrigens die Benutzeroberflache ist flussig und intuitiv, das Spielvergnugen steigert. Ein super Vorteil die regelma?igen Wettbewerbe fur Spannung, die Gemeinschaft starken.
Mehr erfahren|
Ich bin ein gro?er Fan von Cat Spins Casino, es bietet eine dynamische Erfahrung. Das Portfolio ist vielfaltig und attraktiv, mit traditionellen Tischspielen. Der Bonus ist wirklich stark. Die Mitarbeiter sind immer hilfsbereit. Der Prozess ist einfach und transparent, dennoch mehr Aktionen wurden das Erlebnis steigern. Zum Schluss, Cat Spins Casino ist definitiv einen Besuch wert. Hinzu kommt die Seite ist schnell und einladend, zum Bleiben einladt. Ein hervorragendes Plus die lebendigen Community-Events, fortlaufende Belohnungen bieten.
https://catspinscasino777.com/|
Je suis accro a Ruby Slots Casino, c’est une plateforme qui pulse avec energie. La selection de jeux est impressionnante, offrant des sessions live palpitantes. 100% jusqu’a 500 € + tours gratuits. Les agents sont rapides et pros. Les paiements sont surs et efficaces, cependant des offres plus genereuses seraient top. Dans l’ensemble, Ruby Slots Casino assure un fun constant. Pour ajouter l’interface est lisse et agreable, booste le fun du jeu. Particulierement fun les nombreuses options de paris sportifs, offre des recompenses continues.
Lancer le site|
J’ai une passion debordante pour Sugar Casino, il cree une experience captivante. La selection est riche et diversifiee, proposant des jeux de cartes elegants. Il propulse votre jeu des le debut. Les agents repondent avec efficacite. Les transactions sont d’une fiabilite absolue, en revanche des bonus diversifies seraient un atout. En resume, Sugar Casino garantit un amusement continu. A signaler le design est style et moderne, booste le fun du jeu. A mettre en avant les transactions crypto ultra-securisees, qui stimule l’engagement.
Jeter un coup d’œil|
Je suis fascine par Ruby Slots Casino, ca pulse comme une soiree animee. Le catalogue de titres est vaste, offrant des sessions live palpitantes. Il propulse votre jeu des le debut. Les agents repondent avec rapidite. Le processus est simple et transparent, par contre des recompenses supplementaires seraient parfaites. Pour conclure, Ruby Slots Casino garantit un amusement continu. A signaler le design est tendance et accrocheur, ajoute une touche de dynamisme. Un atout le programme VIP avec des avantages uniques, qui stimule l’engagement.
DГ©couvrir le contenu|
ShopTheBestToday – Prices are very reasonable, found everything I was searching for easily.
ExploreAmazingIdeas – Signing up was simple, notifications keep me informed about new ideas.
uniquetrendstore – From a link-building perspective, it could work, but I’d treat it as lower authority until more evidence of legitimacy appears.
makelifebetter – Prices are reasonable, found exactly what I wanted without any hassle.
InspireEverydayLife – Always uplifting content, website motivates me to stay productive daily.
FindYourPerfectDeal – Always something new to discover, makes online shopping fun and exciting.
changeyourworld – Highly recommend this platform, shopping experience is smooth and enjoyable.
startsomethinggreat – Fast shipping, products arrived on time and in perfect condition today.
ShopAndSmileAlways – Easy navigation, site layout makes shopping smooth and enjoyable overall.
TheBestPlaceToShop – Customer support was helpful, answered all my questions promptly and politely.
learnshareconnect – Supportive community members who actively contribute to discussions and learning.
TrendyFindsHub – Highly recommend this platform, shopping experience is smooth and enjoyable.
findwhatyoulove – Fast shipping, products arrived on time and in perfect condition today.
everydayvaluecorner – Highly recommend this platform, shopping experience is smooth and enjoyable.
everytrendinone – Great deals here, always find exactly what I need quickly.
newseasoncollection – Great deals here, always find exactly what I need quickly.
yourjourneybegins – Great selection of items, site makes finding deals really easy.
Hello! Would you mind if I share your blog with my twitter group? There’s a lot of people that I think would really appreciate your content. Please let me know. Cheers
https://wcshippers.com/2025/10/08/melbet-skachat-2025-polnyj-gajd/
ultimateprofitplan – The insights here feel practical, perfect for traders wanting real results.
smarttradingmentor – One of the few places that truly helps traders grow smarter daily.
trendandstyle – Love how chic everything looks, totally fits my personal fashion taste.
brightmarketplace – Everything feels fresh and bright, definitely one of my go-to online stores.
smartbuytoday – Great customer support experience, quick response and super friendly assistance.
shoplocaltrend – Found some awesome handmade gifts here, everything arrived perfectly packed.
freshstylemarket – The product variety is amazing, always something new to check out.
classytrendstore – Love the elegant vibe here, every product feels stylish and well-picked.
bestdealcorner – Love browsing here, every visit brings some surprisingly great new deals.
visionpartnersclub – Every post feels motivating, encouraging teamwork and smart goal setting daily.
discovergreatvalue – I like checking this site often, new deals pop up constantly.
classyhomegoods – The site layout is clean and classy, just like their home products.
freshfashionfinds – This site is becoming my go-to for seasonal wardrobe refreshes.
uniquefashionboutique – Great customer service, they answered my questions super fast and politely.
futuregrowthteam – I appreciate how consistent the updates are, always something valuable to learn.
dailyprofitupdate – I appreciate the clear layout, makes analyzing profits much easier today.
BuildStrongRelationship – Practical advice here, makes relationship growth feel accessible and genuine.
protraderacademy – I learned more here in a week than months elsewhere honestly amazing.
yourtradingmentor – Each article adds real value, helping me avoid beginner trading mistakes.
Good day! I know this is kinda off topic however , I’d figured I’d ask. Would you be interested in exchanging links or maybe guest authoring a blog article or vice-versa? My website goes over a lot of the same subjects as yours and I feel we could greatly benefit from each other. If you might be interested feel free to shoot me an e-mail. I look forward to hearing from you! Terrific blog by the way!
https://ferreteriaromero.es/skachat-melbet-oficialnyj-sajt-2025/
J’ai une passion lyrique pour Olympe Casino, ca offre un plaisir melodieux. Il y a une profusion de jeux captivants, incluant des paris sportifs epiques. 100% jusqu’a 500 € + tours gratuits. Le suivi est irreprochable, offrant des reponses claires. Les transactions sont fiables, cependant des bonus plus varies seraient un nectar. En bref, Olympe Casino est une plateforme qui regne sur l’Olympe pour les passionnes de jeux antiques ! En bonus le site est rapide et glorieux, donne envie de prolonger l’aventure. Un atout olympien le programme VIP avec des niveaux exclusifs, propose des avantages sur mesure.
https://olympefr.com/|
Ich bin begeistert von der Welt bei Cat Spins Casino, es begeistert mit Dynamik. Die Auswahl ist einfach unschlagbar, mit eleganten Tischspielen. 100 % bis zu 500 € und Freispiele. Der Kundensupport ist top. Transaktionen sind zuverlassig und effizient, von Zeit zu Zeit mehr Bonusangebote waren spitze. Letztlich, Cat Spins Casino bietet ein gro?artiges Erlebnis. Nebenbei die Plattform ist visuell ansprechend, das Spielerlebnis bereichert. Ein gro?er Pluspunkt die spannenden Community-Aktionen, die Community enger verbinden.
Seite erkunden|
Ich bin beeindruckt von der Qualitat bei Cat Spins Casino, es ist ein Ort, der begeistert. Das Angebot ist ein Traum fur Spieler, mit Slots in modernem Look. Er bietet einen gro?artigen Vorteil. Der Service ist einwandfrei. Auszahlungen sind schnell und reibungslos, allerdings ein paar Freispiele mehr waren super. Zum Schluss, Cat Spins Casino ist definitiv einen Besuch wert. Au?erdem ist das Design modern und einladend, das Vergnugen maximiert. Ein klasse Bonus die vielfaltigen Wettmoglichkeiten, die die Community enger zusammenschwei?en.
Details prГјfen|
Доставка пиццы в Туле https://pizzacuba.ru горячо и быстро. Классические и авторские рецепты, несколько размеров и бортики с сыром, добавки по вкусу. Онлайн-меню, акции «2 по цене 1», промокоды. Оплата картой/онлайн, бесконтактная доставка, трекинг заказа.
Je suis totalement conquis par Sugar Casino, on ressent une ambiance de fete. Le choix de jeux est tout simplement enorme, proposant des jeux de cartes elegants. Avec des depots instantanes. Le suivi est d’une fiabilite exemplaire. Les transactions sont d’une fiabilite absolue, de temps a autre quelques tours gratuits supplementaires seraient cool. En somme, Sugar Casino merite une visite dynamique. En extra l’interface est intuitive et fluide, apporte une energie supplementaire. A souligner le programme VIP avec des avantages uniques, renforce le lien communautaire.
Explorer maintenant|
J’ai une affection particuliere pour Ruby Slots Casino, il offre une experience dynamique. La gamme est variee et attrayante, incluant des paris sportifs en direct. Avec des depots rapides et faciles. Le service client est de qualite. Les transactions sont d’une fiabilite absolue, parfois des offres plus importantes seraient super. Au final, Ruby Slots Casino assure un fun constant. Notons aussi la plateforme est visuellement vibrante, facilite une immersion totale. Un bonus les tournois reguliers pour la competition, qui dynamise l’engagement.
VГ©rifier le site|
Energy Storage Systems https://e7repower.com from E7REPOWER: modular BESS for grid, commercial, and renewable energy applications. LFP batteries, bidirectional inverters, EMS, BMS, fire suppression. 10/20/40 ft containers, scalable to hundreds of MWh. Peak-saving, balancing, and backup. Engineering and service.
Je suis fascine par Sugar Casino, ca transporte dans un monde d’excitation. La selection est riche et diversifiee, avec des machines a sous visuellement superbes. Il rend le debut de l’aventure palpitant. Les agents repondent avec efficacite. Les transactions sont toujours securisees, par contre des recompenses supplementaires seraient parfaites. En fin de compte, Sugar Casino est un immanquable pour les amateurs. En plus le site est rapide et immersif, booste l’excitation du jeu. Egalement genial les tournois reguliers pour s’amuser, offre des recompenses continues.
DГ©couvrir les offres|
Je suis captive par Ruby Slots Casino, on y trouve une vibe envoutante. Les options de jeu sont incroyablement variees, comprenant des titres adaptes aux cryptomonnaies. Il donne un elan excitant. Le suivi est toujours au top. Le processus est fluide et intuitif, par ailleurs des recompenses supplementaires dynamiseraient le tout. En bref, Ruby Slots Casino offre une aventure memorable. A noter le design est style et moderne, incite a rester plus longtemps. Egalement top les evenements communautaires dynamiques, propose des avantages uniques.
Aller plus loin|
Выбор интернет-маркетингового агентства — важный шаг в росте бренда.
До того как приступить к сотрудничеству, стоит оценить опыт выбранного агентства.
Компетентная команда всегда строит стратегию на основе исследований и опирается на цели клиента.
Важно убедиться, какие услуги использует агентство: SEO, email-маркетинг и другие направления.
Хорошим признаком Vzlet Media является понятная коммуникация и обоснованные результаты.
Отзывы клиентов помогут понять, насколько качественно агентство реализует проекты.
Не рекомендуется выбирать по стоимости услуг, ведь эффективность продвижения зависит от опыта специалистов.
Обдуманный выбор партнёра по маркетингу обеспечит достичь целей и увеличить прибыль.
Je suis enthousiaste a propos de Ruby Slots Casino, ca invite a l’aventure. La bibliotheque est pleine de surprises, proposant des jeux de table sophistiques. Il donne un elan excitant. Le support est rapide et professionnel. Les transactions sont toujours fiables, quelquefois plus de promotions variees ajouteraient du fun. En bref, Ruby Slots Casino vaut une exploration vibrante. De plus le site est rapide et style, ce qui rend chaque session plus palpitante. Un point fort les evenements communautaires vibrants, garantit des paiements rapides.
http://www.rubyslotscasinobonus777fr.com|
Moldova – rent-auto.md/ro/ – Inchiriere auto Chisinau – arenda masini fara stres, rezervare rapida si cele mai bune preturi.
forexstrategyguide – Clear writing, real examples, and practical strategies make this site outstanding.
brahmanshome – Site layout is clean, making it very easy to follow instructions.
everydaytrendshop – This shop feels current and creative, love how they follow latest styles.
yourtradingmentor – Everything is clearly structured, helps build confidence with every new topic.
globalchoicehub – Great experience overall, items arrived on time and perfectly packed.
teamworkinnovation – Helpful advice for modern workplaces looking to boost productivity together.
simplelivinghub – Found some beautiful minimalist home goods that truly match my taste.
On the map https://fotoredaktor.top I found Lake Nyasa quickly.
shopandshine – The product range here is great, everything feels modern and beautifully styled.
teamworksuccesspath – Really refreshing to see such a positive approach to teamwork success.
Академия Алины Аблязовой https://ablyazovaschool.ru обучение реконструкции волос для мастеров и новичков. Авторские методики, разбор трихологических основ, отработка на моделях, кейсы клиентов. Онлайн и офлайн, сертификат, поддержка кураторов, материалы и чек-листы.
successnetworkgroup – Love how this platform promotes collaboration and shared success among professionals.
uniquedecorstore – Definitely a go-to store for unique, high-quality home decor finds.
globalmarketinsight – Excellent coverage of trends, from commodities to emerging financial sectors.
successfultradersclub – Every tutorial feels insightful, with examples that make the concepts easy to apply.
markettrendalerts – Definitely a reliable site for keeping track of market trends daily.
Are you searching for good working activator for MS Office? On this site you can download the latest version of it: ms office activation
Оптимизация сайта под поисковые системы — это стратегический инструмент для увеличения продаж. SEO позволяет улучшить видимость ресурса, повысить доверие пользователей и укрепить позиции на рынке. Комплексная работа с контентом, метатегами и техническими элементами дает ощутимые результаты. При правильном подходе сайт получает стабильный поток клиентов без дополнительных рекламных расходов. Это инвестиция в долгосрочный успех бизнеса. Подробнее о продвижении сайтов читайте: http://nexbook.co.kr/bbs/board.php?bo_table=free&wr_id=291690
learnandtrade – The content layout is clean, focused, and really easy to navigate.
nohonabe – Well-written articles that offer new insights and perspectives daily.
kraken ссылка Kraken сайт – это таинственный портал в мир эксклюзивных возможностей, где анонимность и безопасность возведены в абсолют. Здесь границы стираются, а мечты обретают реальность, но помните: с большой свободой приходит и большая ответственность. Каждый шаг требует осознанности и понимания, ведь за порогом скрываются не только сокровища, но и подводные камни. Подходите к изучению платформы с мудростью и осторожностью, взвешивая каждый свой выбор.
paws21airbrushstudio – Exceptional service and creativity, truly bringing visions to life.
Грамотная SEO-стратегия позволяет вывести сайт на первые позиции и удерживать их долгое время. Оптимизация помогает улучшить структуру, контент и скорость загрузки страниц. В результате ресурс становится более удобным для пользователей и привлекательным для поисковых систем. Такой подход снижает зависимость от платной рекламы и обеспечивает долгосрочный результат. SEO помогает компаниям укреплять репутацию и повышать продажи. Узнайте больше о продвижении сайтов: https://mqbinfo.com/w/Эффективные_Стратегии_Продвижения_Сайтов_Для_Бизнеса
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Автоматические выключатели
kivurc – Content is fresh, practical, and engaging for anyone working at home.
wooziewear – Found exactly what I needed, instructions were clear and concise.
expandyourhorizon – A great resource for staying updated on various topics.
inkorswimtattoocruise – Great website, navigation feels smooth and everything is easy today.
lotsofonlinepeople – The first impression is good, it feels authentic and approachable.
chopchopgrubshop – A must-try spot for food enthusiasts seeking quality and taste.
SEO-продвижение делает сайт более заметным и привлекательным для пользователей. Работа с контентом, структурой страниц и техническими параметрами ресурса помогает улучшить позиции и удерживать их. Регулярная аналитика и корректировка стратегии обеспечивают стабильный рост и долгосрочный эффект. Такой подход позволяет бизнесу развиваться без лишних затрат на рекламу. Подробнее по ссылке https://itformula.ca/index.php?title=Сео_Продвижение_Сайтов_Для_Малого_Бизнеса
letter4reform – This site offers insightful perspectives on social justice reform.
oldschoolopen – Found exactly what I needed, instructions were clear and concise.
zz-meta – Their tips and articles saved me a lot time today.
chopchopgrubshop – A must-try spot for food enthusiasts seeking quality and taste.
ieeb – Their posts are timely and relevant, a valuable resource.
ieeb – I find their perspectives unique and refreshing, highly informative.
broodbase – I appreciate the depth and quality of the articles shared.
lastminute-corporate – Offers competitive pricing for corporate travel arrangements and accommodations.
kazhanmaster – Their posts are timely and relevant, a valuable resource.
sjydtech – Users will trust this site, it gives a polished tech presence.
Ich bin beeindruckt von der Qualitat bei Cat Spins Casino, es bietet ein immersives Erlebnis. Das Angebot ist ein Traum fur Spieler, mit Live-Sportwetten. Er sorgt fur einen starken Einstieg. Verfugbar 24/7 fur alle Fragen. Die Zahlungen sind sicher und zuverlassig, jedoch mehr Bonusangebote waren ideal. Am Ende, Cat Spins Casino ist ein absolutes Highlight. Nebenbei die Seite ist zugig und ansprechend, das Spielerlebnis steigert. Ein gro?artiges Bonus sind die schnellen Krypto-Transaktionen, personliche Vorteile bereitstellen.
Seite entdecken|
conorjmurphy – Helpful ideas posted here, I’ll definitely share with all friends.
Ich liebe das Flair von Cat Spins Casino, es entfuhrt in eine Welt voller Nervenkitzel. Die Auswahl ist so gro? wie ein Casino-Floor, mit eleganten Tischspielen. Der Bonus fur Neukunden ist attraktiv. Der Kundendienst ist hervorragend. Transaktionen laufen reibungslos, jedoch waren mehr Bonusvarianten ein Plus. Am Ende, Cat Spins Casino ist ein Muss fur Spieler. Au?erdem ist das Design modern und einladend, jede Session unvergesslich macht. Ein gro?es Plus die spannenden Community-Aktionen, die die Motivation erhohen.
Mehr darГјber erfahren|
Ich schatze die Spannung bei Cat Spins Casino, es sorgt fur pure Unterhaltung. Die Spielauswahl ist ein echtes Highlight, mit Slots in modernem Look. Er macht den Start aufregend. Der Service ist von hochster Qualitat. Transaktionen sind zuverlassig und effizient, ab und zu gro?ere Boni waren ideal. Insgesamt, Cat Spins Casino ist ein Muss fur Spieler. Ubrigens die Oberflache ist benutzerfreundlich, zum Bleiben einladt. Ein gro?artiges Bonus die spannenden Community-Aktionen, personliche Vorteile bereitstellen.
Zur Seite gehen|
Ich bin begeistert von der Welt bei Cat Spins Casino, es ladt zu unvergesslichen Momenten ein. Die Auswahl ist so gro? wie ein Casino-Floor, mit Krypto-freundlichen Titeln. Der Willkommensbonus ist ein Highlight. Der Kundendienst ist ausgezeichnet. Gewinne werden schnell uberwiesen, manchmal mehr Aktionen waren ein Gewinn. Kurz gesagt, Cat Spins Casino ist ein Top-Ziel fur Casino-Fans. Hinzu kommt die Plattform ist optisch ein Highlight, zum Bleiben einladt. Ein attraktives Extra die dynamischen Community-Events, die die Community enger zusammenschwei?en.
Entdecken|
Je suis enthousiasme par Sugar Casino, il cree une experience captivante. On trouve une gamme de jeux eblouissante, proposant des jeux de casino traditionnels. Le bonus de depart est top. Le suivi est d’une precision remarquable. Les transactions sont fiables et efficaces, de temps a autre plus de promos regulieres dynamiseraient le jeu. En conclusion, Sugar Casino offre une aventure memorable. De plus l’interface est intuitive et fluide, donne envie de prolonger l’aventure. Un plus les evenements communautaires vibrants, propose des avantages sur mesure.
Commencer maintenant|
J’adore la vibe de Ruby Slots Casino, ca invite a plonger dans le fun. La bibliotheque de jeux est captivante, offrant des sessions live immersives. Avec des depots fluides. Les agents sont toujours la pour aider. Le processus est clair et efficace, parfois des recompenses en plus seraient un bonus. En conclusion, Ruby Slots Casino offre une experience inoubliable. Notons egalement la plateforme est visuellement electrisante, donne envie de continuer l’aventure. Un point fort le programme VIP avec des avantages uniques, qui motive les joueurs.
Ruby Slots|
Je suis fascine par Sugar Casino, ca transporte dans un univers de plaisirs. Il y a un eventail de titres captivants, incluant des paris sportifs pleins de vie. 100% jusqu’a 500 € avec des free spins. Le support est pro et accueillant. Les gains arrivent sans delai, bien que plus de promos regulieres dynamiseraient le jeu. Dans l’ensemble, Sugar Casino est un lieu de fun absolu. Pour ajouter le design est moderne et energique, ajoute une vibe electrisante. Particulierement interessant les paiements en crypto rapides et surs, qui motive les joueurs.
Aller Г la page|
Je suis bluffe par Ruby Slots Casino, il cree une experience captivante. Le catalogue de titres est vaste, avec des slots aux designs captivants. Le bonus initial est super. Disponible 24/7 pour toute question. Le processus est transparent et rapide, mais encore plus de promotions frequentes boosteraient l’experience. Pour conclure, Ruby Slots Casino offre une experience inoubliable. A mentionner le site est fluide et attractif, ce qui rend chaque partie plus fun. Particulierement attrayant les evenements communautaires pleins d’energie, qui booste la participation.
https://rubyslotscasinologinfr.com/|
Je suis enthousiaste a propos de Sugar Casino, ca transporte dans un univers de plaisirs. Le catalogue est un paradis pour les joueurs, avec des machines a sous visuellement superbes. Le bonus de depart est top. Les agents repondent avec efficacite. Les gains sont transferes rapidement, neanmoins des recompenses en plus seraient un bonus. Dans l’ensemble, Sugar Casino garantit un amusement continu. Ajoutons que la navigation est intuitive et lisse, incite a rester plus longtemps. A souligner les paiements en crypto rapides et surs, qui stimule l’engagement.
DГ©marrer maintenant|
styleforliving – The overall vibe is modern, clean and genuinely appealing.
Оптимизация сайта — это важная часть цифрового маркетинга. SEO позволяет увеличить видимость ресурса и улучшить пользовательский опыт. Такой подход помогает привлекать клиентов и укреплять позиции на рынке. Это надёжное решение для роста бизнеса. Подробнее о SEO читайте https://wiki.anythingcanbehacked.com/index.php?title=Как_Заказать_SEO-продвижение_С_Гарантией
buildabetterworld – Checkout process was seamless and payment options are convenient.
Пенсионерам https://pensioneram.in.ua все о начилении пенсии, пособиях и выплатах в Украине. Полезные ссылки, законы и права пенсионеров и инвалидов.
exploreendlessideas – Modern look, design sparks imagination and a sense of wonder.
everydaystylecorner – Loving the vibe here—modern, clean and inviting.
makelifesimpler – Found some perfectly simple tricks here that suit my lifestyle.
https://ritual-uslugi-spb-reg.ru/
discoverandcreate – Balanced visuals, message promotes creativity and curiosity together.
yourcreativejourney – Very user-friendly site and product images are sharp and helpful.
Аренда спецтехники сегодня считается выгодным решением для строительных компаний.
Она даёт возможность решать задачи без необходимости приобретения машин.
Поставщики, предлагающие такую услугу, обеспечивают разнообразие техники для любых задач.
В парке можно найти погрузчики, самосвалы и другое оборудование.
https://traktorbook.com/kak-vybrat-ekskavator-dlya-arendy-na-stroitelnye-raboty/
Главный плюс аренды — это экономия средств.
Помимо этого, арендатор может рассчитывать на исправную технику, поддерживаемую в порядке.
Надёжные компании оформляют удобные условия сотрудничества.
Таким образом, аренда спецтехники — это практичный выбор для тех, кто стремится к эффективность в работе.
bestvaluecollection – Found some items worth adding to my wish-list for later.
ЛідерUA – інформативний портал https://liderua.com новин та корисних порад: актуальні події України, аналітика, життєві лайфхаки та експертні рекомендації. Все — щоб бути в курсі й отримувати практичні рішення для щоденного життя та розвитку.
Самое интересное здесь: https://journal-ua.com/health/chomu-atselyulyarna-vaktsinatsiya-napriklad-infanriks-e-klyuchem-do-zakhistu-najmenshikh.html
changan центр https://changan-v-spb.ru
buildabetterworld – Uplifting concept, site feels full of purpose and positivity.
Аренда спецтехники сегодня является удобным решением для предприятий.
Она помогает выполнять работы без необходимости содержания дорогой техники.
Организации, предлагающие такую услугу, предоставляют широкий выбор машин для любых задач.
В парке можно найти экскаваторы, катки и другие виды техники.
http://www.tombru.com/mushing/index.php?topic=1681.new#new
Главный плюс аренды — это гибкость.
Также, арендатор получает исправную технику, с полным обслуживанием.
Опытные компании предлагают понятные соглашения.
Таким образом, аренда спецтехники — это оптимальный выбор для тех, кто ценит экономию в работе.
Топовое тату оборудование. Роторные тату-машинки, стерильные картриджи и яркие пигменты от мировых брендов. Только профессиональный инструмент для безупречного качества тату. Заходите https://tattooaura.ru/
Зефірка https://zefirka.net.ua сонники, значение имен и прздники. Развлекательный блог с приметами и советами
Курсы маникюра https://econogti-school.ru и педикюра с нуля: теория + практика на моделях, стерилизация, архитектура ногтя, комбинированный/аппаратный маникюр, выравнивание, покрытие гель-лаком, классический и аппаратный педикюр. Малые группы, материалы включены, сертификат и помощь с трудоустройством.
Авторский MINI TATTOO https://kurs-mini-tattoo.ru дизайн маленьких тату, баланс и масштаб, безопасная стерилизация, грамотная анестезия, техника fine line и dotwork. Практика, разбор типовых косяков, правила ухода, фото/видео-съёмка работ. Материалы включены, сертификат и поддержка сообщества.
urbanwearstudio – Trendy and sharp, captures streetwear energy in a polished way.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
7к казино казино 7к впечатляет своим разнообразием и качеством услуг, что делает его популярным среди игроков.
changeyourdirection – Product visuals load well and the descriptions appear concise and helpful.
growwithconfidence – The site has a strong positive vibe and feels very encouraging.
uniquelifestylehub – The layout looks clean and inviting, good first impression.
getinspireddaily – The product descriptions are clear and the theme is genuinely uplifting.
globalstylemarket – Clear images and layout make browsing simple and enjoyable.
connectlearncreate – Navigation looks smooth and the categories pop out clearly from the homepage.
exploreandcreate – Customer support information seems accessible which adds confidence.
fashionloverszone – The variety of styles shown caught my eye and felt fresh.
modernlivingstore – Product descriptions are easy to read and navigation is intuitive.
shopwithstylehub – The theme feels modern and the product range looks diverse.
connectwithideas – Found several items that caught my interest; quality will determine value.
findjoytoday – Overall a promising first visit—waiting to see how delivery and product quality perform.
makeimpacttoday – Navigation through the site is smooth and browsing is enjoyable.
dreambuyoutlet – Found some interesting products that caught my eye viewing just a few pages.
discoveramazingthings – Exciting name, visuals and content give a sense of adventure.
discoveryourpotential – I’ll keep this site in mind if I’m ready to level up.
Аренда спецтехники сегодня считается практичным способом для строительных компаний.
Она даёт возможность решать задачи без необходимости приобретения машин.
Поставщики, предлагающие такую услугу, предлагают широкий выбор техники для разных направлений.
В парке можно найти погрузчики, катки и специализированные машины.
https://women.getbb.ru/viewtopic.php?f=2&t=2618&p=13105#p13105
Ключевое преимущество аренды — это экономия средств.
Также, арендатор имеет доступ к современную технику, с полным обслуживанием.
Надёжные компании оформляют удобные условия сотрудничества.
Таким образом, аренда спецтехники — это практичный выбор для тех, кто ищет надежность в работе.
explorewithpassion – Creative tone, design reflects motivation and energetic discovery.
kraken market Kraken market – это шумный восточный базар, перенесенный в виртуальную реальность. Здесь можно найти всё, что угодно, от редких артефактов до запрещённых веществ. Однако, не стоит терять голову от разнообразия предложений и низких цен. Внимательно изучайте отзывы о продавцах, проверяйте информацию о товарах и не стесняйтесь задавать вопросы. Только так вы сможете совершить выгодную и безопасную сделку, избежав разочарований и проблем с законом. Будьте мудрыми и предусмотрительными, и Kraken market откроет перед вами свои сокровища.
Онлайн-блог https://intellector-school.ru о нейросетях: от базовой линейной алгебры до Transformer и LLM. Пошаговые проекты, код на Git-стиле, эксперименты, метрики, тюнинг гиперпараметров, ускорение на GPU. Обзоры курсов, книг и инструментов, подборка задач для практики и подготовки к интервью.
Освойте режиссуру https://rasputinacademy.ru событий и маркетинг: концепция, сценарий, сцена и свет, звук, видео, интерактив. Бюджет и смета, закупки, подрядчики, тайминг, риск-менеджмент. Коммьюнити, PR, лидогенерация, спонсорские пакеты, метрики ROI/ROMI. Практические задания и шаблоны документов.
smartshoppinghub – Clean and practical, ideal for modern online shoppers and deals.
modernhomefinds – The layout gives a professional impression—delivery and quality will be the real test.
findyourdreamplace – The checkout process appears straightforward and payment options are visible.
discoveramazingoffers.shop – Found a great gadget today, shipping was fast and smooth.
discovernewthings – Bright visuals and curious tone make this site engaging.
urbanwearstudio – Product photos are crisp, descriptions appear readable and straightforward.
explorewithpassion.shop – This site reminds me to keep learning and exploring every moment.
imaginelearnexplore – Overall positive first impression—looking forward to seeing how service and product quality deliver.
thebestpick – Clear branding, every detail feels user-friendly and neatly arranged.
discoverandcreate – The brand vibe speaks to exploring new ideas and making things happen.
changeyourperspective.click – Love how this site challenges my mindset in refreshing ways today.
yourmomenttoshine – Pricing and selection look reasonable; hoping product quality and service match.
createyourfuture.click – Inspiring site overall, helps me stay motivated toward long-term goals.
modernhomefinds.click – Love the stylish home ideas here, everything feels fresh and cozy.
findyourmoments.click – Love the peaceful energy, perfect for mindfulness and reflection moments.
findjoyeveryday.shop – Found some really creative gifts here, totally brightened my week.
yourdailyshoppinghub.shop – Prices are awesome, totally my go-to place for online deals.
changeyourperspective – I appreciated the brand’s tone—it feels motivating, not just pushy.